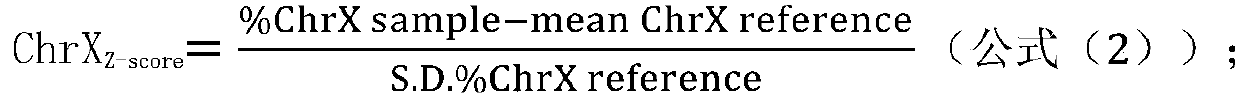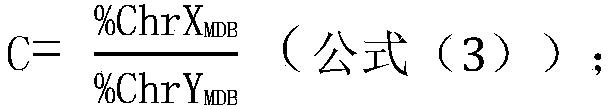Kit for non-invasively detecting whether fetal chromosome to be tested is aneuploid and special probe set thereof
An aneuploidy and chromosome technology, applied in the medical field, can solve the problems of complex data analysis, long cycle and high cost of whole genome sequencing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Example 1. Preparation of a kit for non-invasive detection of whether the fetal chromosome to be tested is aneuploid
[0168] 1. Preparation of probe set
[0169] 1. According to the specific region of human chromosome 13, the specific region of human chromosome 18, the specific region of human chromosome 21, the specific region of human chromosome X and the specific region of human chromosome Y, design and synthesize 500 probe. Each probe consists of 108 nucleotides, including DNA fragment 1, DNA fragment 2 and DNA fragment 3 from the 5' end to the 3' end. The nucleotide sequence of DNA fragment 1 is 5'-GACTACATGGGACAT-3'. The nucleotide sequence of DNA fragment 3 is 5'-GGAACCTACGACGTA-3'. Both DNA fragments 2 consist of 78 nucleotides. The DNA fragment 2 is a specific region of human chromosome 13, a specific region of human chromosome 18, a specific region of human chromosome 21, a specific region of human chromosome X or a part of a specific region of human chro...
Embodiment 2
[0182]Example 2, the establishment of a method for non-invasive prenatal detection of whether the fetus to be tested is suffering from chromosomal aneuploidy using the kit prepared in Example 1
[0183] After a lot of experiments, the inventors of the present invention have established a method for non-invasive prenatal detection of whether the fetus to be tested is suffering from chromosomal aneuploidy using the kit prepared in Example 1. Specific steps are as follows:
[0184] 1. Extraction of cell-free DNA from plasma of pregnant women to be tested
[0185] 1. Use EDTA anticoagulant tubes to collect 10 mL of peripheral blood from pregnant women to be tested, centrifuge at 1600 g at 4°C for 15 min, and collect supernatant 1 (placed in a low-absorption centrifuge tube).
[0186] 2. After completing step 1, take the supernatant 1, centrifuge at 12,000 rpm for 5 minutes, and collect the supernatant 2 (placed in a low-absorption centrifuge tube). Supernatant 2 is plasma.
[0...
Embodiment 3
[0280] Example 3. Using the kit prepared in Example 1 for non-invasive prenatal detection of whether the fetus to be tested suffers from chromosomal aneuploidy
[0281] The samples to be tested were peripheral blood samples of 1 high-risk pregnant woman with trisomy 13 (numbered 7), peripheral blood samples of 2 high-risk pregnant women with trisomy 18 (numbered 1 and 6), and 2 samples of high-risk pregnant women with trisomy 18. 21-trisomy syndrome Tang screening high-risk pregnant women peripheral blood samples (numbered 2 and 3), 1 case of superfemale syndrome (specifically XXX) Tang screening high-risk pregnant women peripheral blood samples (numbered 4), 1 case of Cranfer Peripheral blood samples (number 5) of high-risk pregnant women with Turner syndrome (specifically XXY) and 1 case of high-risk pregnant women with Turner syndrome (specifically XO) (number 9) and 1 case of supermale Syndrome (specifically XYY) peripheral blood samples (number 8) of Tang screening high-r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com