A long afterglow luminescent airgel and its preparation method
A long afterglow luminescence and aerogel technology, applied in luminescent materials, chemical instruments and methods, etc., can solve the problems of high cost and poor dispersion, and achieve the effects of good uniformity, enhanced skeleton, and controllable sample density.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
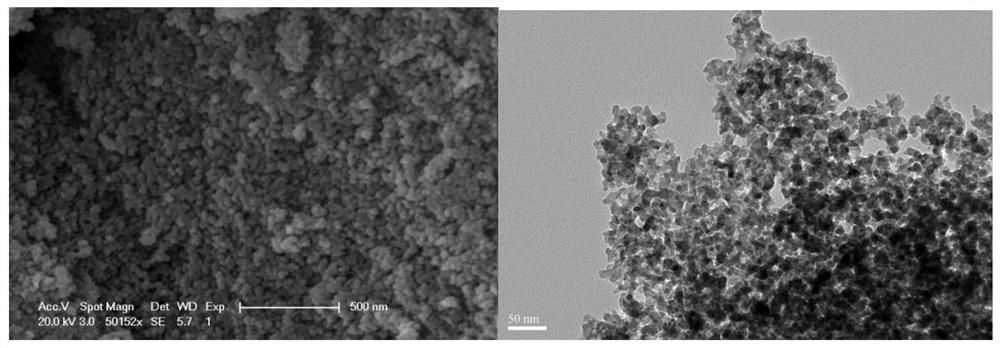
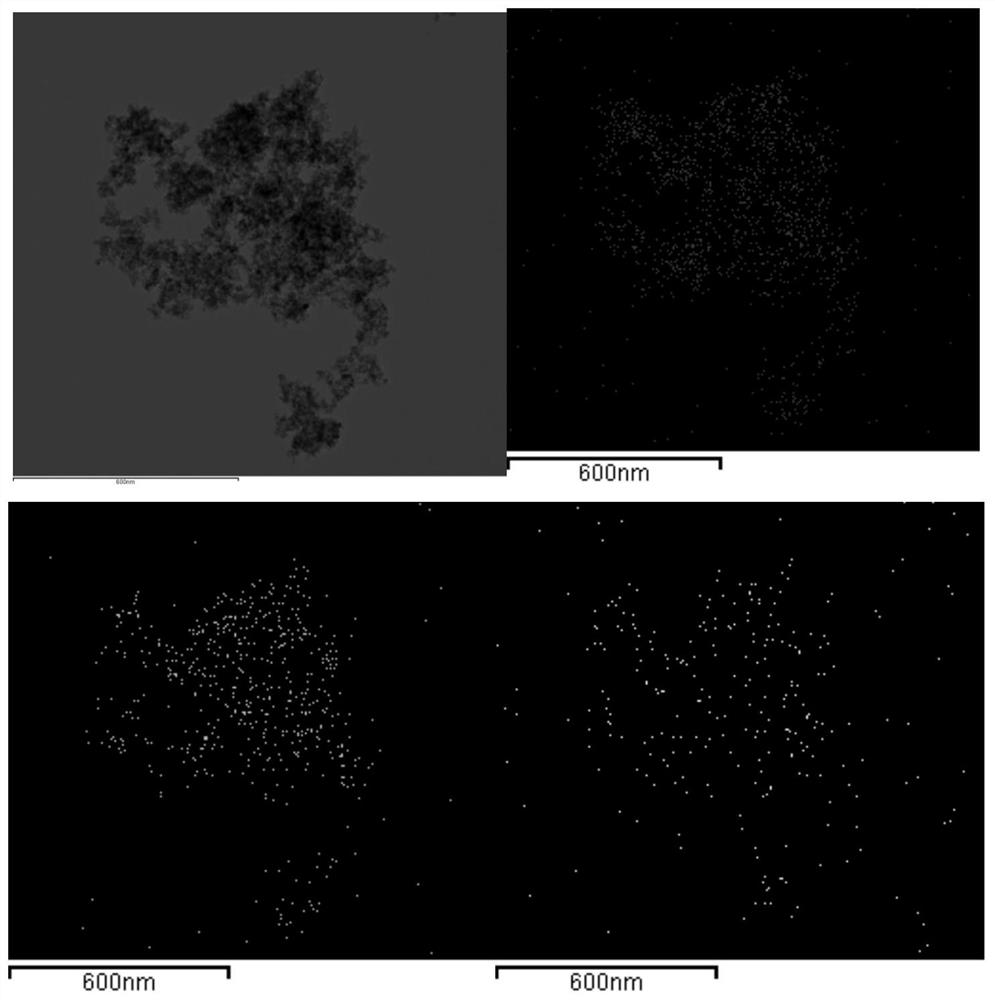
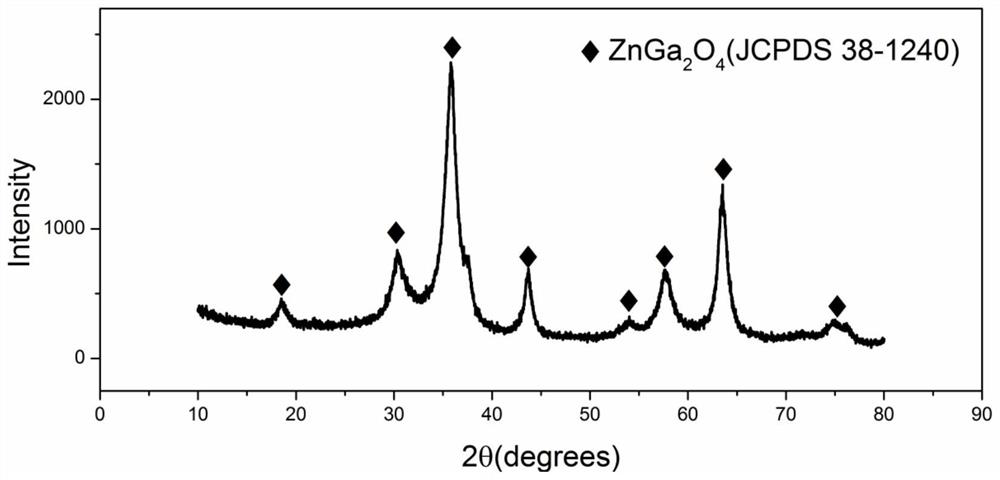
[0033] 2.98g zinc nitrate [Zn(NO 3 ) 2 ·6H 2 O] and 2.56g gallium nitrate [Ga(NO 3 ) 3 ·xH 2 O] was dissolved in 30 ml of ethanol, stirred evenly, then added 0.03 g of chromium nitrate [Cr(NO 3 ) 3 9H 2 O] Stir until the solution is homogeneous. Add 2ml of deionized water and 2.31ml of polyacrylic acid (PAA) to the mixed solution, stir at room temperature for 30 minutes, then add 3.78ml of propylene oxide (PO), and leave it at room temperature until it gels after 3 minutes. Spread a layer of ethanol on the upper surface of the obtained wet gel for aging. After three days, replace the ethanol solvent at room temperature, 8-12 hours each time, replace 3-5 times, and then perform supercritical carbon dioxide drying to reach the supercritical condition. With a temperature of 40°C and a pressure of 9MPa, ZnGa was obtained after drying 2 o 4 :Cr 3+ airgel. The airgel was calcined in a muffle furnace at 800°C for 2 hours to obtain ZnGa 2 o 4 :Cr 3+ Long-lasting glowing...
Embodiment 2
[0036] Adopt the same preparation method as Example 1, the difference is that the preparation conditions used are different, specifically comprising the following steps:
[0037] (1) Dissolve 0.01mol zinc nitrate hexahydrate, 0.01mol gallium nitrate hydrate and 0.00005mol chromium nitrate nonahydrate in ethanol, mix well, then add 0.1mol deionized water and 0.03mol polyacrylic acid, stir at 300r / min Down stirring 30min, obtain mixed solution;
[0038] (2) Add 0.0067mol propylene oxide to the mixed solution, stir for 30min at a stirring rate of 300r / min, and then let it stand until it turns into a gel;
[0039] (3) Cover the surface of the gel with a layer of ethanol for aging, replace the ethanol every 8 hours, and replace it 5 times, and then perform supercritical carbon dioxide drying at a temperature of 43 ° C, a pressure of 7.5 MPa, and a time of 4 hours to obtain an airgel Crude;
[0040] (4) Calcining the crude airgel at 600° C. for 4 hours to obtain the long-lasting l...
Embodiment 3
[0043] Adopt the same preparation method as Example 1, the difference is that the preparation conditions used are different, specifically comprising the following steps:
[0044] (1) Dissolve 0.01mol zinc nitrate hexahydrate, 0.01mol gallium nitrate hydrate and 0.0001mol chromium nitrate nonahydrate in ethanol, mix well, then add 0.13mol deionized water and 0.045mol polyacrylic acid, stir at 1000r / min Down stirring 15min, obtain mixed solution;
[0045] (2) Add 1.5mol propylene oxide to the mixed solution, stir for 15min at a stirring speed of 1000r / min, and then let it stand until it turns into a gel;
[0046] (3) Cover the surface of the gel with a layer of ethanol for aging, replace the ethanol every 12 hours, and replace it 3 times, and then perform supercritical carbon dioxide drying at a temperature of 38 ° C, a pressure of 9.5 MPa, and a time of 3 hours to obtain an airgel Crude;
[0047] (4) Calcining the crude airgel at 1200° C. for 2 hours to obtain the long-lastin...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap