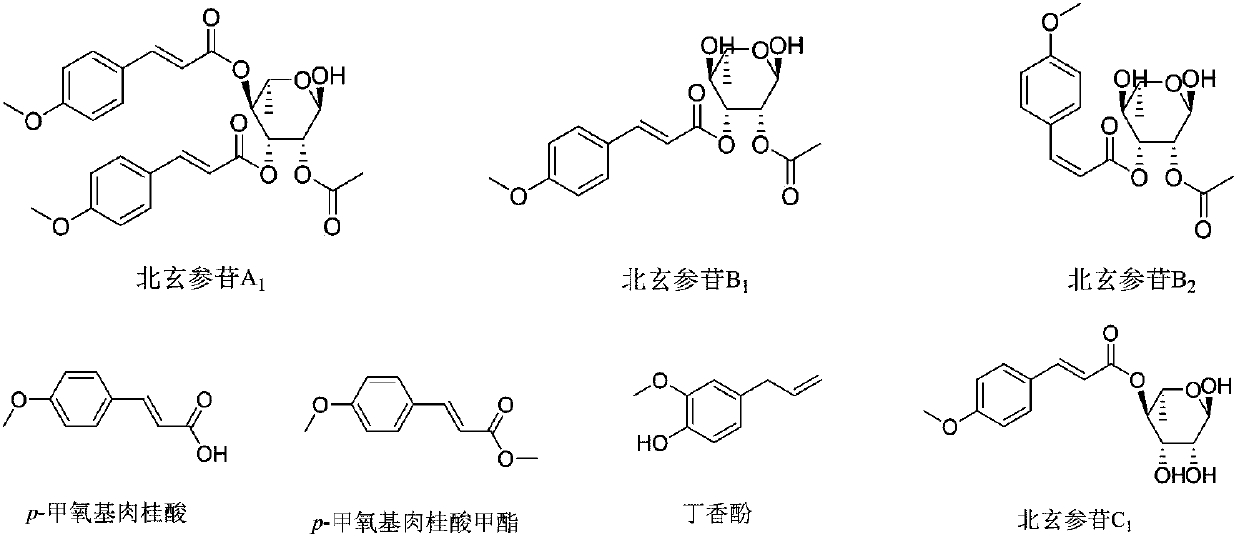
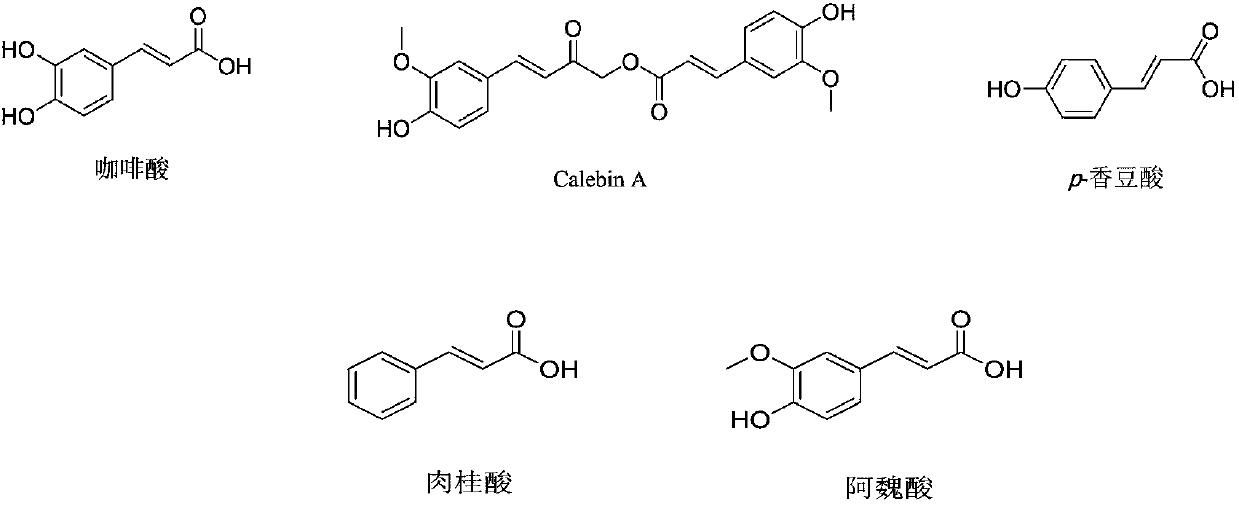
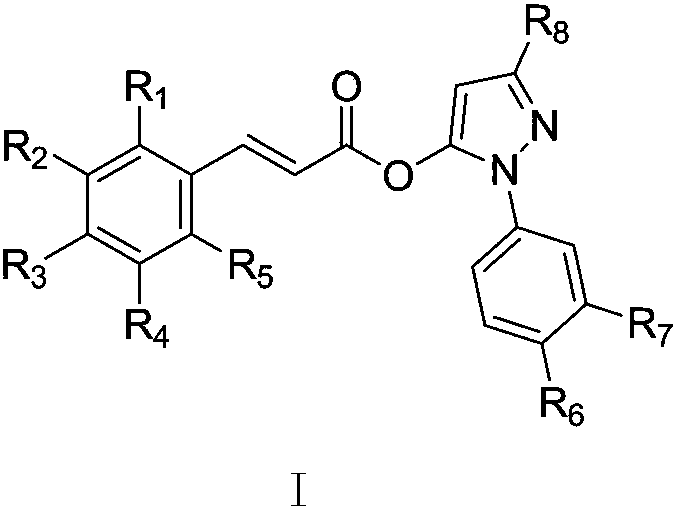
Phenylacrylate derivative and application thereof as neuroprotective drug
A technology of phenylacrylate and phenylacrylic acid, which is applied in the field of phenylacrylate derivatives, can solve the problems of low oral bioavailability, only injection administration, unstable physical and chemical properties, etc., and achieve high neuroprotective activity and acute toxicity Low, ideal effect of pharmacokinetic characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 (E)-3-methyl-1-phenyl-1H-pyrazole-5-(3-(4-hydroxyphenyl)) acrylate (T-1)
[0038] 4-Hydroxyphenylacrylic acid (5 mmol, 1.0 eq) was dissolved in 40 mL of dichloromethane at room temperature. Oxalyl chloride (6.5 mmol, 1.3 eq) was slowly added dropwise to the solution at 0-5°C. After the dropwise addition was completed, it was moved to room temperature for 8h. After the reaction, vacuum-dry to obtain 4-hydroxyphenylacryloyl chloride.
[0039]Dissolve phenylhydrazine hydrochloride (7.5mmol, 1.0eq) and triethylamine (11.3mmol, 1.5eq) in 20mL absolute ethanol at room temperature. The reaction solution was heated to 50° C., and ethyl acetoacetate (7.5 mmol, 1.0 eq) was slowly added dropwise. After the addition was complete, the reaction was refluxed for 24 hours. After completion of the reaction, vacuum-dry to obtain 3-methyl-1-phenyl-2-pyrazolin-5-one.
[0040] 3-Methyl-1-phenyl-2-pyrazolin-5-one (7.5 mmol, 1.5 eq) was dissolved in 30 mL ...
Embodiment 2
[0042] Embodiment 2 (E)-3-methyl-1-phenyl-1H-pyrazole-5-(3-(4-hydroxyl-3-methoxyphenyl)) acrylate (T-2) and its salt preparation
[0043] 4-Hydroxy-3-methoxyphenylacrylic acid (5 mmol, 1.0 eq) was dissolved in 40 mL of dichloromethane at room temperature. Oxalyl chloride (6.5 mmol, 1.3 eq) was slowly added dropwise to the solution at 0-5°C. After the dropwise addition was completed, it was moved to room temperature for 8h. After the reaction, vacuum-dry to obtain 4-hydroxy-3-methoxyphenylacryloyl chloride.
[0044] Dissolve phenylhydrazine hydrochloride (7.5mmol, 1.0eq) and triethylamine (11.3mmol, 1.5eq) in 20mL absolute ethanol at room temperature. The reaction solution was heated to 50° C., and ethyl acetoacetate (7.5 mmol, 1.0 eq) was slowly added dropwise. After the addition was complete, the reaction was refluxed for 24 hours. After completion of the reaction, vacuum-dry to obtain 3-methyl-1-phenyl-2-pyrazolin-5-one.
[0045] 3-Methyl-1-phenyl-2-pyrazolin-5-one (7....
Embodiment 3
[0055] Example 3 Preparation of (E)-3-methyl-1-phenyl-1H-pyrazole-5-(3-(4-methoxyphenyl))acrylate (T-3) and its salts
[0056] 4-Methoxyphenylacrylic acid (5 mmol, 1.0 eq) was dissolved in 40 mL of dichloromethane at room temperature. Oxalyl chloride (6.5 mmol, 1.3 eq) was slowly added dropwise to the solution at 0-5°C. After the dropwise addition was completed, it was moved to room temperature for 8h. After the reaction, vacuum-dry to obtain 4-methoxyphenylacryloyl chloride.
[0057] Dissolve phenylhydrazine hydrochloride (7.5mmol, 1.0eq) and triethylamine (11.3mmol, 1.5eq) in 20mL absolute ethanol at room temperature. The reaction solution was heated to 50° C., and ethyl acetoacetate (7.5 mmol, 1.0 eq) was slowly added dropwise. After the addition was complete, the reaction was refluxed for 24 hours. After completion of the reaction, vacuum-dry to obtain 3-methyl-1-phenyl-2-pyrazolin-5-one.
[0058] 3-Methyl-1-phenyl-2-pyrazolin-5-one (7.5 mmol, 1.5 eq) was dissolved in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com