1-O-alkyl genipin and preparation method and application thereof
A technology of alkyl genipin and genipin, applied in nervous system diseases, organic chemistry, drug combination, etc., can solve the problem of weak activity of genipin derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The invention provides a kind of preparation method of 1-O-alkylgenipin, comprises the following steps:
[0041] Mix raw material A, lower carbon alcohol, Lewis acid catalyst and polar organic solvent (hereinafter referred to as the first polar organic solvent) to carry out alkoxylation reaction to obtain 1-O-alkyl genipin, and the raw material A includes jing Nepin and / or 10-O-Piv-1-O-NHCl 3 Genipin, the 10-O-Piv-1-O-NHCl 3 Genipin is shown in formula I:
[0042]
[0043] In the present invention, unless otherwise specified, the raw materials used are commercially available products well known to those skilled in the art.
[0044] In the present invention, the raw material A includes genipin and / or 10-O-Piv-1-O-NHCCl 3 Genipin, more preferably comprising genipin or 10-O-Piv-1-O-NHCl 3 Genipin.
[0045] In the present invention, the 10-O-Piv-1-O-NHCCl 3 The preparation method of genipin preferably comprises the following steps:
[0046] 10-O-Piv-genipin of str...
Embodiment 1
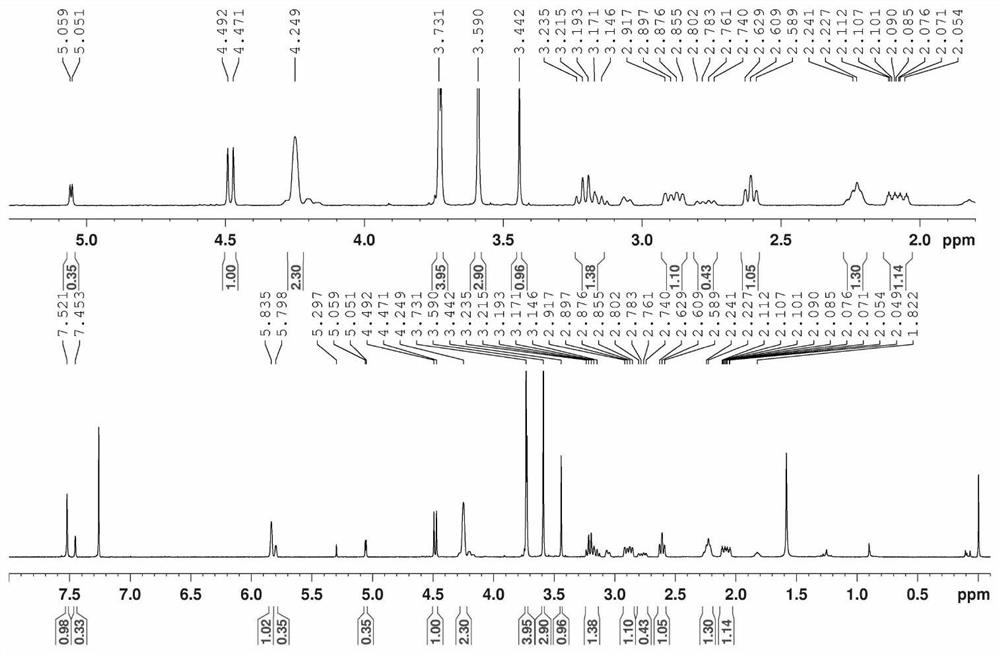
[0114] In a 50mL round bottom flask, add genipin 0.2g (0.88mmol), methanol (2.65mmol), Molecular sieves (0.05g) and 10mL DMC were slowly added dropwise (pseudo-dropping speed 0.5mL / min) boron trifluoride diethyl ether (0.15mL, 1.33mmol) into the reaction system under the protection of nitrogen, and at -30°C, After reacting for 8h hours, the reaction system was poured into 50mL ice water to quench, and extracted 3 times with dichloromethane, each time the amount of dichloromethane was 50ml), and the combined organic phase after 3 extractions was washed with 100mL saturated brine to Neutral, with anhydrous Na 2 SO 4 After drying, it was concentrated under reduced pressure, and purified by silica gel column chromatography (the eluent was a mixed solvent of ethyl acetate and petroleum ether, and the volume ratio of the two was 1:3) to obtain a light yellow oil, which was 1-O-methyl Genipin, NMR spectrum such as figure 1 shown.
Embodiment 2~14
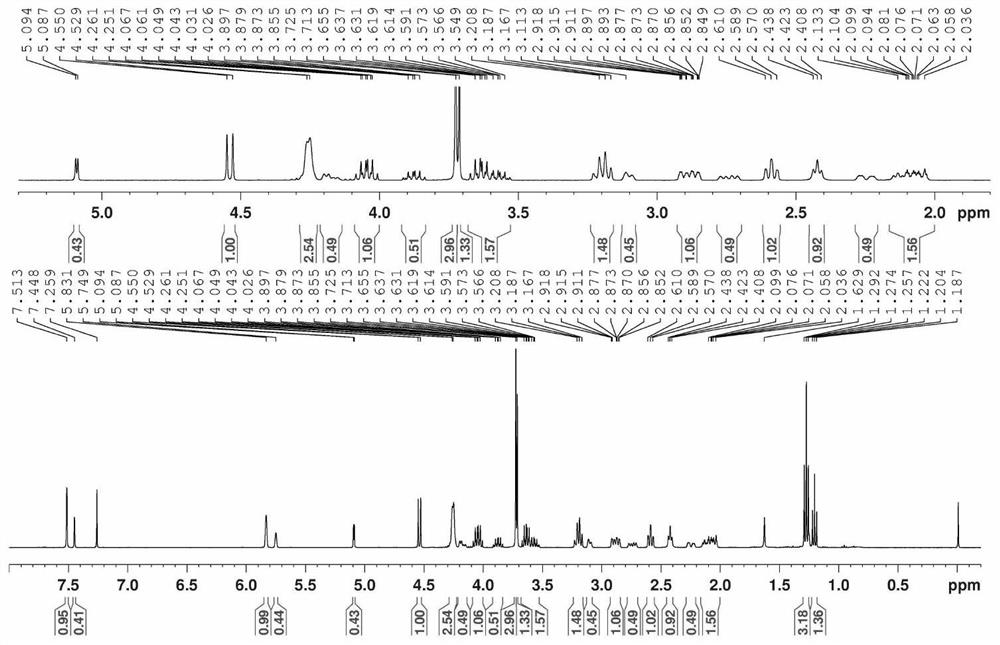
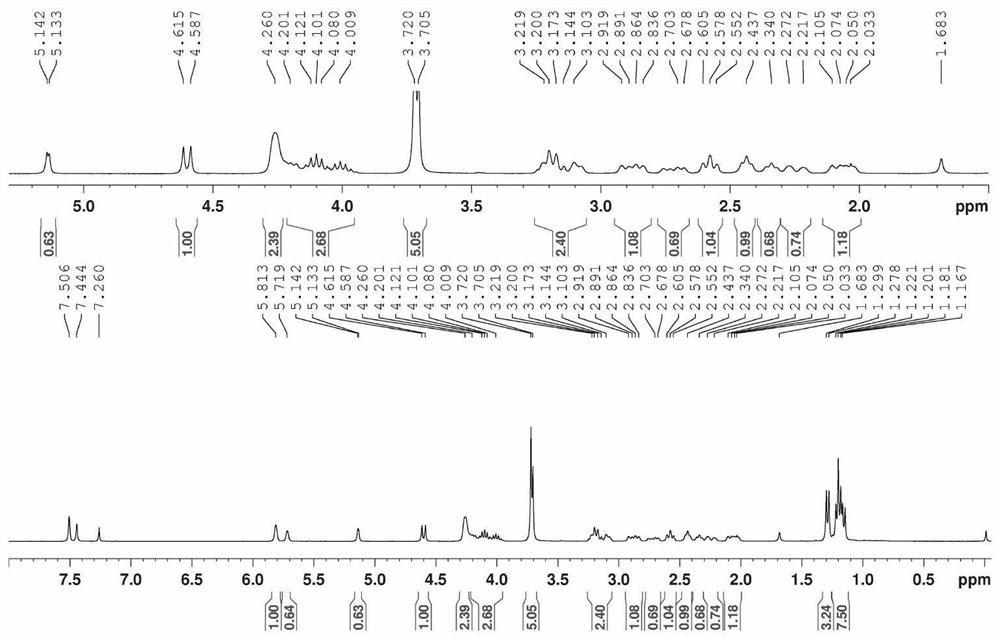
[0116] Embodiment 1~14 implementation process is identical with embodiment 1, and the kind of reaction raw material, reaction temperature, polar organic solvent that embodiment 1~14 uses is listed in table 1, and the 1-O-alkane that embodiment 1~14 obtains The isolated yield of genipin, the molar ratio of 1S-O-alkylgenipin and 1R-O-alkylgenipin ( c1H-NMR determination) are listed in Table 1.
[0117] The reaction equation among the embodiment 1~14 is shown in formula V:
[0118]
[0119] The reaction condition parameter and the result of table 1 embodiment 1~14
[0120]
[0121]
[0122] The 1-O-methylgenipin of embodiment 1~8 preparation 1 H-NMR is:
[0123] 1-O-Methylgenipin: 1 H-NMR (CDCl 3 )δ:2.05-2.26(2H,m),2.59-2.63(2H,m),2.74-2.92(2H,m),3.04-3.23(2H,m),3.43(3H,s),3.59(3H, s),3.73(3H,s),4.19(1H,d,J=13.2Hz),4.25(2H,s),4.26(1H,d,J=13.2Hz),4.49(1H,d,J=8.0 Hz), 5.07(1H,d,J=3.2Hz), 5.80(1H,s), 5.84(1H,s), 7.46(1H,s), 7.52(1H,s).
[0124] Implementation of 1-O...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com