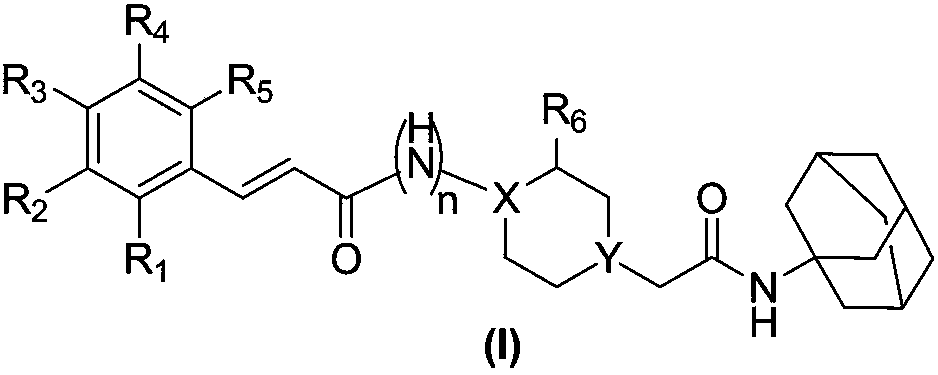
Application of acetyl amantadine piperazine (piperidine) compound as cerebral neuroprotective agent
A kind of technology of acetylamantadine piperazine and compound, which is applied in the field of brain nerve protective agent, and achieves the effect of ideal pharmacokinetic characteristics, strong protective activity and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of Example 1N-(1-(2-(adamantyl-1-amino)-2-acetyl)piperidine-4-substituted)cinnamide (I-1) and salts thereof
[0072] Prepare according to general synthesis method V-1, and the specific operation is as follows:
[0073] Preparation of intermediate chloroacetylamantadine:
[0074] Dissolve 1-adamantanamine (4.87g, 32.2mmol) in 50mL of DCM, under nitrogen protection, slowly add triethylamine (4.94mL, 35.5mmol) dropwise at 0°C, stir for 15min, then slowly add chloroacetyl chloride ( 2.82mL, 37.4mmol), maintain this temperature, continue to stir for 2h. After the reaction was completed, water was added to the reaction liquid to separate the layers, and the organic layer was washed with dilute hydrochloric acid and saturated sodium bicarbonate in sequence, and the concentrated product of the organic layer was collected and recrystallized with petroleum ether / ethyl acetate to obtain acetylamantadine chloride, 6.28 g, The rate is 85.6%. ESI-MS(m / z):228.2[M+H]+. ...
Embodiment 2
[0089]Example 2 (E)-N-(1-(2-(adamantyl-1-amino)-2-acetyl)piperidine-4-substituted)-3-(2-methoxyphenyl)propene Preparation of amides (I-2) and their salts
[0090] According to the general synthesis method V-1 operation, the target product I-20.43g was obtained with a yield of 55.4%. ESI-MS[M+H] + : m / z=452.1, 1 H NMR (400MHz, DMSO-d6) δppm: 7.99 (d, 1H, J = 7.6Hz), 7.24 (d, 1H, J = 15.6Hz), 7.07 (s, 1H), 6.88 (s, 2H), 6.47 (d,1H,J=15.6Hz),3.81(m,4H),2.80(m,4H),2.01(s,4H),1.93(d,6H,J=2.4Hz),1.78(d,4H, J=10.4Hz), 1.63(s, 6H), 1.43(m, 4H).
[0091] The preparation of compound 1-2 hydrobromide
[0092] Using compound I-2 (2.0 mmol) and 5% hydrobromic acid aqueous solution (2.1 mmol) as raw materials, the preparation method of compound T-1 hydrobromide was used to obtain 0.9 g of white I-2 hydrobromide solid.
[0093] Preparation of compound I-1 oxalate
[0094] Compound I-2 (2.0mmol) and oxalic acid dihydrate (2.4mmol) were added to ethanol (10mL), refluxed to dissolve, and...
Embodiment 3
[0095] Example 3 (E)-N-(1-(2-(adamantyl-1-amino)-2-acetyl)piperidine-4-substituted)-3-(4-hydroxyl-3-methoxy Preparation of phenyl)acrylamide (I-3) and its salt
[0096] According to the general synthesis method V-1 operation, the target product I-30.73g was obtained with a yield of 65.4%. ESI-MS[M+H] + : m / z=468.2, 1 H NMR (400MHz, DMSO-d6) δppm: 8.11 (d, 1H, J = 7.6Hz), 7.24 (d, 1H, J = 15.6Hz), 6.88 (s, 2H), 6.43 (d, 1H, J = 15.6Hz), 3.81(m, 4H), 2.81(m, 4H), 2.21(s, 4H), 1.93(d, 6H, J=2.4Hz), 1.78(d, 4H, J=10.4Hz), 1.63 (s,6H), 1.43(m,4H).
[0097] Preparation of compound 1-3 acetate
[0098] Add compound I-3 (0.3g) and acetic acid (0.8mmol) to ethanol (10mL), reflux to dissolve, cool and reduce pressure to concentrate the acid to obtain a solid, beat with acetone and cool to precipitate a white solid, filter to obtain 0.22g of white I-3 Acetate solid.
[0099] Preparation of compound 1-3 sulfate
[0100] Add compound I-3 (0.3 g) and sulfuric acid (0.8 mmol) into wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com