A kind of method for cultivating hepatitis E virus in vitro
A hepatitis E virus, in vitro culture technology, applied in the directions of viruses, viruses/phages, biochemical equipment and methods, etc., can solve the problem that cannot use cancer cell line HEV vaccine research and development and production, cannot be stably continuously subcultured, cannot be real. Reaction-related symptoms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Hepatitis E virus cultured in vitro by MDCK cells
[0025] 1. Take out MDCK cells (purchased from ATCC in the United States) from liquid nitrogen and immediately put them into warm water at 37°C to melt the cell suspension quickly; centrifuge at 1500 rpm for 5 minutes, then transfer to 10cm 3 Add DMEM culture solution (purchased from GIBCO Invitrogen Corporation) containing 10% newborn bovine serum by volume to the cell culture flask, culture at 37°C, and after growing into a dense monolayer, wash with PBS (purchased from GIBCO Invitrogen Corporation) Wash the cells, digest with trypsin-EDTA (purchased from GIBCO Invitrogen Corporation), add the above culture medium, and place at 37°C, 5% CO 2 Continue culturing in the incubator;
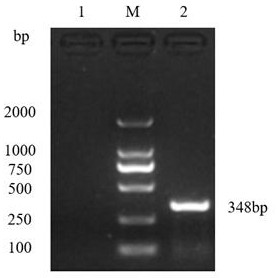
[0026] 2. Prepare a 10% weight-volume ratio of PBS (pH7.4) feces suspension from the collected HEV-positive feces for HEV isolation, shake vigorously to emulsify the feces, centrifuge at 12,000g for 10 minutes at 4°C, and colle...
Embodiment 2
[0028] Embodiment 2: Continuous culture of hepatitis E virus in MDCK cells in vitro
[0029] 1. Take out MDCK cells (purchased from ATCC in the United States) from liquid nitrogen and immediately put them in warm water at 37°C to melt the cell suspension quickly; centrifuge at 1500 rpm for 5 minutes, then transfer to 10cm 3Add DMEM (purchased from GIBCO Invitrogen Corporation) culture solution containing 10% newborn bovine serum (purchased from GIBCO Invitrogen Corporation) to the cell culture flask, and culture at 37°C. Wash the cells, digest with trypsin-EDTA (purchased from GIBCO Invitrogen Corporation), add the above culture medium, and place at 37°C, 5% CO 2 Continue culturing in the incubator;
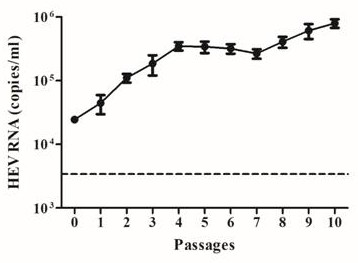
[0030] 2. Prepare a PBS (pH7.4) feces suspension with a weight-volume ratio of 15% from the collected HEV-positive feces for HEV isolation, shake vigorously to emulsify the feces, centrifuge at 12,000g for 10 minutes at 4°C, and collect the supernatant. Filter and sterilize thr...
Embodiment 3
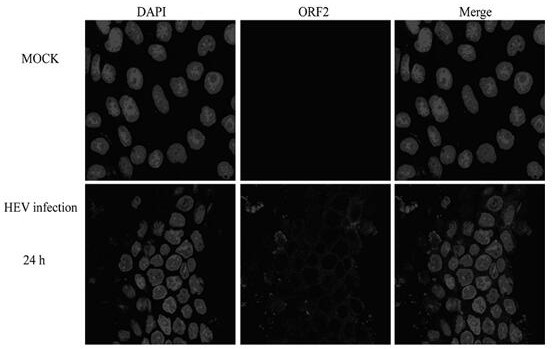
[0034] Example 3: Observation of HEV-infected MDCK cells by fluorescence confocal microscope
[0035] 1. Take out MDCK cells (purchased from ATCC, USA) from liquid nitrogen and immediately put them into warm water at 37°C to melt the cell suspension quickly; centrifuge at 1500 rpm for 5 minutes, then transfer to 10cm 3 Add DMEM (purchased from GIBCO Invitrogen Corporation) culture solution containing 10% newborn bovine serum by volume to the cell culture flask, culture at 37°C, and wash with PBS (purchased from GIBCO Invitrogen Corporation) after growing into a dense monolayer Cells were digested with trypsin-EDTA (purchased from GIBCO Invitrogen Corporation), added to the above culture medium, and placed at 37°C, 5% CO 2 Continue culturing in the incubator;
[0036] 2. Prepare a PBS (pH7.4) feces suspension with a weight-volume ratio of 15% from the collected HEV-positive feces for HEV isolation, shake vigorously to emulsify the feces, centrifuge at 12,000g for 10 minutes at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com