Device and method for detecting concentration of divalent iron ions in real time
A real-time detection and ion concentration technology, applied in the field of analysis and detection, can solve problems such as influence and inability to capture detail changes well, and achieve the effect of real-time detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
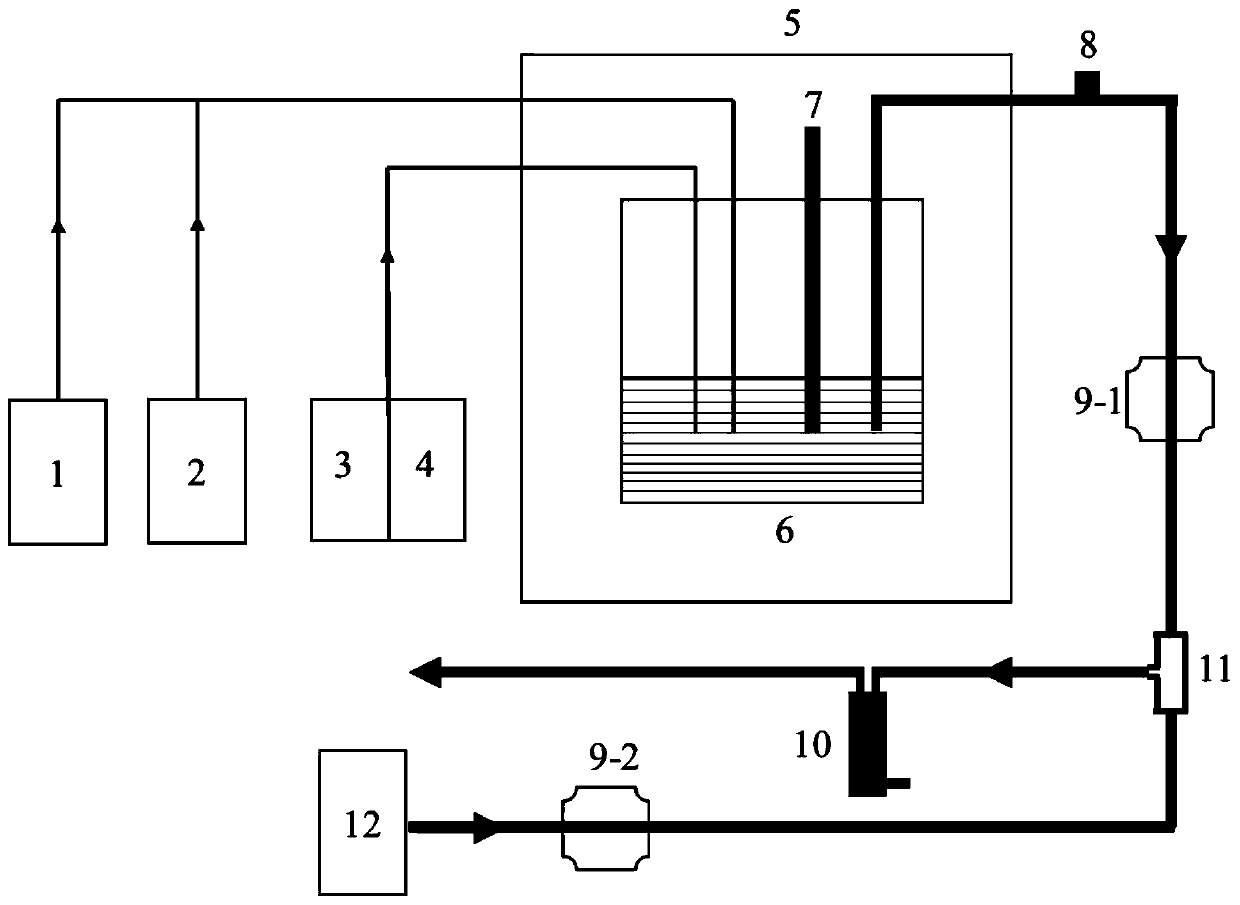
[0039] N was introduced into the closed reactor 2 and CO 2 Mixed gas, control the ratio of mixed gas to 8:1, keep its pH at 6.5, set the temperature in the multifunctional reaction box to 25°C, and keep it away from light. The dissolved organic matter solution (DOM, concentration 30mg / L) and the ferrous ion solution (concentration is 0.5mg / L) in the ferrous ion reagent injector were passed into the airtight reactor. After the experiment started, the DOM solution flow rate gradually increased from 0mL / s to 5mL / s, the ferrous ion reagent solution flow control is 5mL / s, carries out the binding reaction of DOM and ferrous ion, obtains complex product and remaining ferrous ion solution; The iron ion solution is controlled by a peristaltic pump into the color reactor, and reacts with o-phenanthroline solution (concentration: 0.5mg / L), and the flow ratio of the two is 3:1 to obtain a color product; real-time measurement shows The concentration of the colored product was obtained to...
Embodiment 2
[0041] N was introduced into the closed reactor 2 and CO 2 Mixed gas, control the ratio of mixed gas to 5:1, keep its pH at 5.5, set the temperature in the multifunctional reaction box to 20°C, and in the state of natural light, put the nitrobenzene solution (concentration of 0.01 μg / L) and the ferrous ion solution (concentration is 0.1mg / L) in the ferrous ion reagent injector are passed in the airtight reactor, carry out ferrous ion reduction nitrobenzene reaction, after experiment begins, The flow of ferrous ion solution increases gradually from 0mL / s to 10mL / s, and the flow of nitrobenzene solution is controlled to be 2mL / s to obtain the reacted product and the remaining ferrous ion solution; The iron ion solution is controlled by a peristaltic pump into the color reactor, and reacts with o-phenanthroline solution (concentration: 0.5mg / L), and the flow ratio of the two is 3:1 to obtain a color product; real-time measurement shows The concentration of the colored product w...
Embodiment 3
[0043] N was introduced into the closed reactor 2 and CO 2 Mixed gas, control the ratio of mixed gas to 4:1, keep its pH at 5.5, set the temperature in the multifunctional reaction box to 25°C, and put the domestic sewage (COD concentration of 300mg / L) and the ferrous ion solution (concentration of 0.2mg / L) in the ferrous ion reagent injector are passed into the multifunctional reaction box, and the flow rate of the two is controlled at 10mL / s, and the ferrous ion solution induced by ultraviolet light is carried out. Ion reaction, after the experiment started, the light intensity was gradually increased from 0W to 500W, and the remaining ferrous ion solution after the induction reaction was obtained; the remaining ferrous ion solution after the induction reaction was controlled by a peristaltic pump into the color reactor In the process, carry out color reaction with o-phenanthroline solution (concentration is 0.5mg / L), and the flow ratio of the two is 1:1 to obtain the colo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com