Clinical test data inspection doubt management system and management method
A technology for clinical trials and data inspection, applied in the field of clinical trial data supervision and management, can solve problems such as difficulty in online inspection of clinical trial data, and achieve the effect of improving the degree of intelligence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
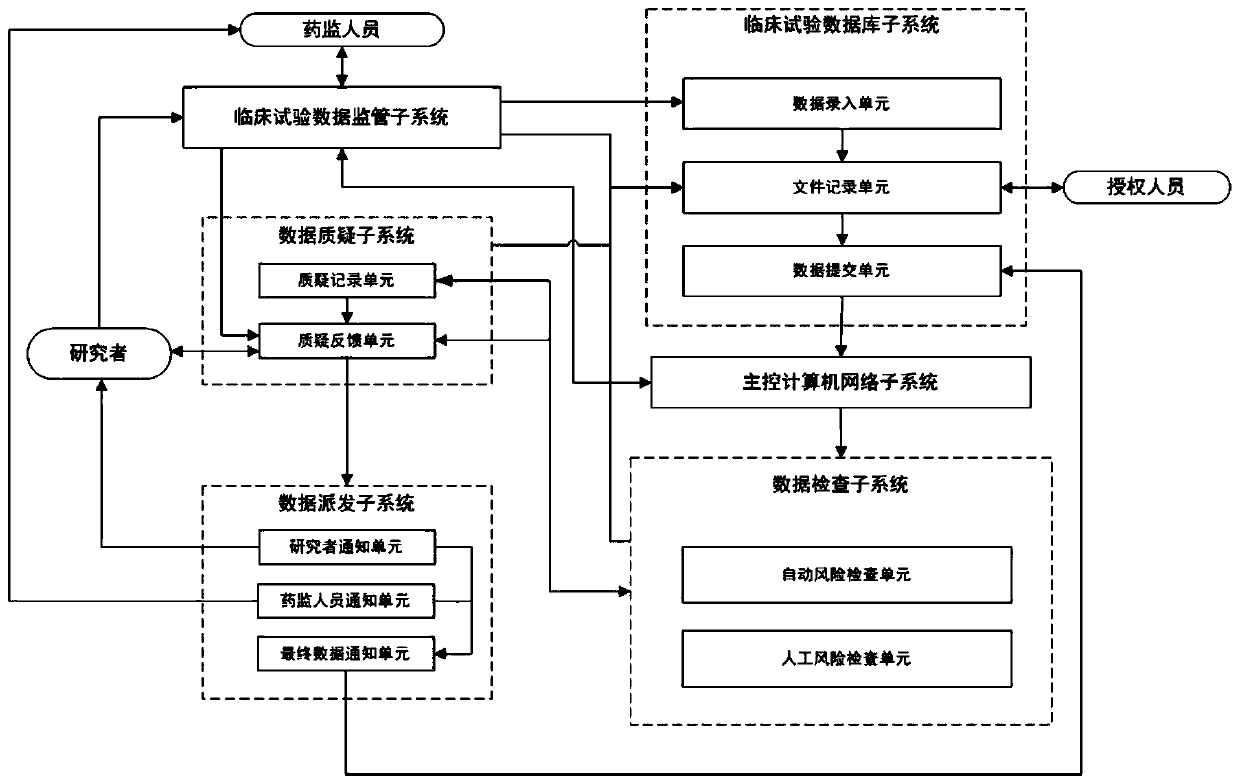
[0037] see figure 1 , a schematic structural diagram of a clinical trial data inspection query management system provided by an embodiment of the present invention. The clinical trial data inspection query management system provided by the embodiment of the present invention includes a clinical trial data supervision subsystem, a clinical trial database subsystem, a main control computer network subsystem, a data inspection subsystem, a data query subsystem and a data distribution subsystem.
[0038] The clinical trial database subsystem collects the clinical drug trial data entered by researchers through the clinical trial data supervision subsystem, and performs data entry and file records for the clinical drug trial data. Inspection and management; the clinical drug trial data entered in the clinical trial database subsystem will be transmitted to the main control computer network subsystem through the Internet; the connection between the main control computer network subsy...
Embodiment 2
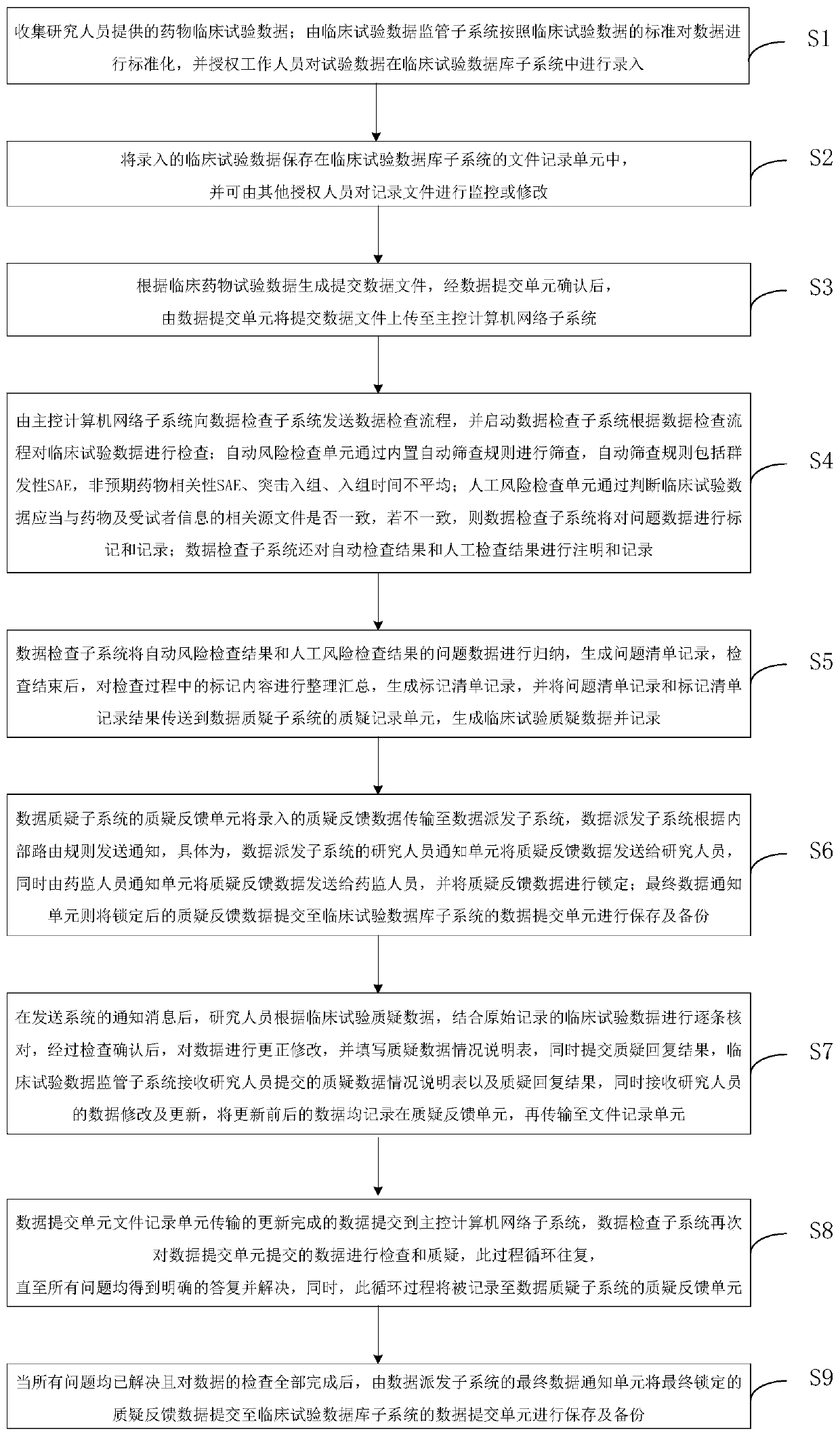
[0065] see figure 2 , a flow chart of the clinical trial data inspection challenge management method provided by the embodiment of the present invention. The clinical trial data inspection query management method provided by the embodiment of the present invention includes the following steps:
[0066] (1) Collect the drug clinical trial data provided by the investigator; the clinical trial data supervision subsystem standardizes the data according to the clinical trial data standards, and authorizes the staff to enter the trial data in the clinical trial database subsystem;
[0067] (2) Save the entered clinical trial data in the file recording unit of the clinical trial database subsystem, and monitor or modify the record files by authorized personnel;
[0068] (3) Generate and submit data files based on clinical drug trial data, and after being confirmed by the data submission unit, the data submission unit uploads the submitted data files to the main control computer net...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com