Alepterolic acid derivative and preparation method and application thereof
A technology for fenback fern acid and derivatives, which is applied in the field of fenback fern acid derivatives and their preparation, can solve the problems of short course of treatment and significant curative effect in advanced glaucoma, and achieves simple and convenient synthesis process, high synthesis efficiency and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
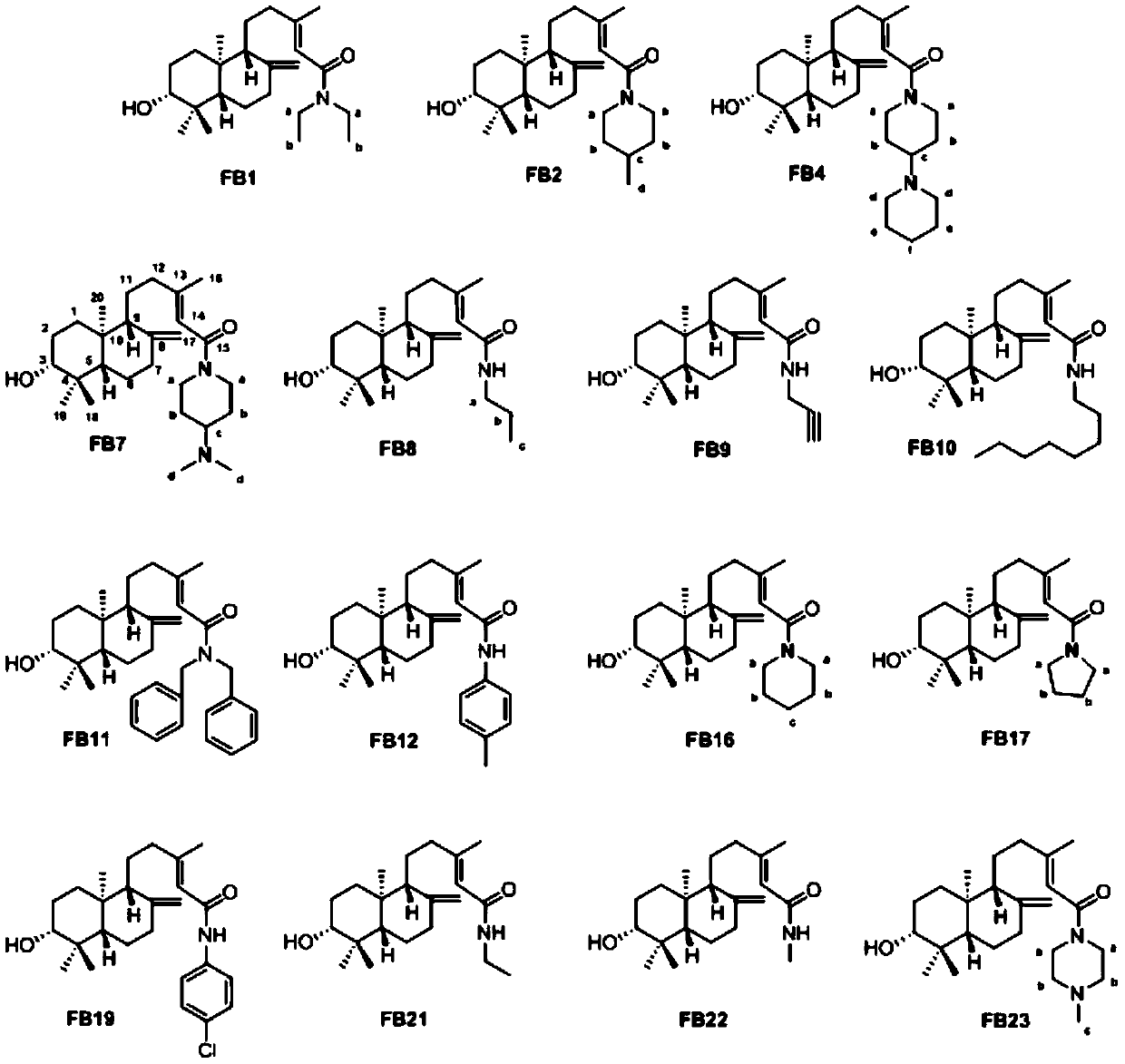
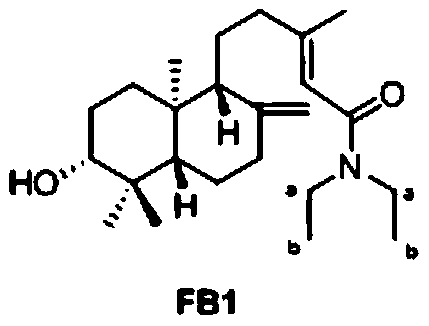
[0056] Example 1
[0057] (E)-N,N-Diethyl-5-([1R,4aS,6R,8aS]-6-hydroxy-5,5,8a-trimethyl-2-methylenedecalin-1- Preparation of 3-methylpent-2-enamide (Compound FB1):
[0058] Dissolve 32mg Pteris acid with 5mL dichloromethane, add 19mL diisopropylethylamine, 47mg condensing agent HATU, and finally add 22mL diethylamine, mix well and stir at room temperature for 2h. After the reaction is complete, add water to terminate the reaction Then it was extracted with dichloromethane, the dichloromethane phase was evaporated to dryness by rotating, and the target compound was obtained by separation and purification.
[0059] The NMR data are as follows: 1 H NMR(500MHz, CDCl 3 )δ5.78-5.74(m,1H,14-H), 4.88-4.84(m,1H,17-H), 4.53(s,1H,17-H),3.46-3.36(m,2H,a-C H2 ), 3.32(q,J=7.1Hz,2H,a-C H2 ), 3.24 (dt, J = 10.1, 3.9 Hz, 1H, 3-H), 2.41 (ddd, J = 12.8, 4.1, 2.4 Hz, 1H, 7-H), 2.23 (ddd, J = 14.0, 9.9, 4.2Hz,1H,12-H),1.95(td,J=14.9,13.6,6.0Hz,2H,7-H,12-H),1.90-1.88(d,J=0.9Hz,3H,16-C H3 ),1.81-1.48(m,7...
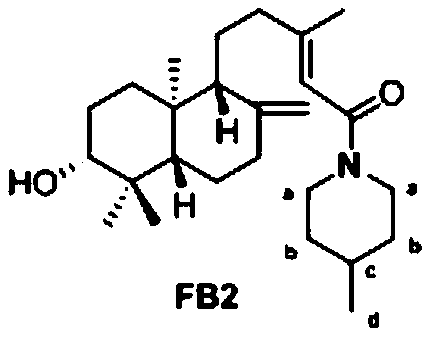
Example Embodiment
[0061] Example 2
[0062] (E)-5-([1R,4aS,6R,8aS]-6-hydroxy-5,5,8a-trimethyl-2-methylenedecalin-1-yl)-3-methyl- Preparation of 1-(4-methylpiperazin-1-yl)pent-2-en-1-one (compound FB2):
[0063] Dissolve 32mg Pteris acid with 5mL of dichloromethane, add 19mL of diisopropylethylamine, 47mg of condensation agent HATU, and finally add 12mg of 4-methylpiperidine, mix well and stir at room temperature for 2h, after the reaction is complete The reaction was terminated by adding water and then extracted with dichloromethane. The dichloromethane phase was rotated and evaporated to dryness, and the target compound was obtained by separation and purification.
[0064] The NMR data are as follows: 1 H NMR(500MHz, CDCl 3 )δ5.70(s,1H,14-H), 4.85(d,J=1.6Hz,1H,17-H),4.57(d,J=13.3Hz,1H,aH),4.52(d,J= 1.6Hz, 1H, 17-H), 3.88 (d, J = 13.2Hz, 1H, aH), 3.23 (dd, J = 12.0, 4.3 Hz, 1H, 3-H), 2.95 (t, J = 12.5 Hz ,1H,aH),2.58(t,J=12.8Hz,1H,aH), 2.40(dd,J=9.3,2.6Hz,1H,7-H),2.22(ddd,J=13.9,9.7,3.8Hz ,1H,12-H...
Example Embodiment
[0066] Example 3
[0067] (E)-1-([1,4'-piperidine]-1'-yl)-5-([1R,4aS,6R,8aS]-6-hydroxy-5,5,8a-trimethyl- Preparation of 2-methylene decalin-1-yl)-3-methylpent-2-en-1-one (compound FB4):
[0068] Dissolve 32mg Pteris acid with 5mL of dichloromethane, add 19mL of diisopropylethylamine, 47mg of condensing agent HATU, and finally add 25.3mg of 4-piperidinylpiperidine, mix well and stir at room temperature for 2h, wait for reaction After completion, water was added to terminate the reaction, and then extracted with dichloromethane. The dichloromethane phase was rotated and evaporated to dryness, separated and purified to obtain the target compound.
[0069] The NMR data are as follows: 1 H NMR(500MHz, CDCl 3 )δ5.71(s,1H,14-H), 4.85(s,1H,17-H), 4.67(d,J=12.8Hz,1H,aH), 4.52(s,1H,17-H), 3.96(d,J=13.5Hz,1H,aH), 3.23(dd,J=11.7,4.4Hz,1H,3-H), 2.95(t,J=12.8Hz,1H,aH), 2.59-2.37( m,7H,aH,7-H,cH,2×dC H2 ), 2.22(ddd,J=14.1,9.6,4.1Hz,1H,12-H),1.99-1.33(m,23H,7-H,12-H,1-H,16-CH 3 ,6-CH 2 ,9-H,11-CH...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap