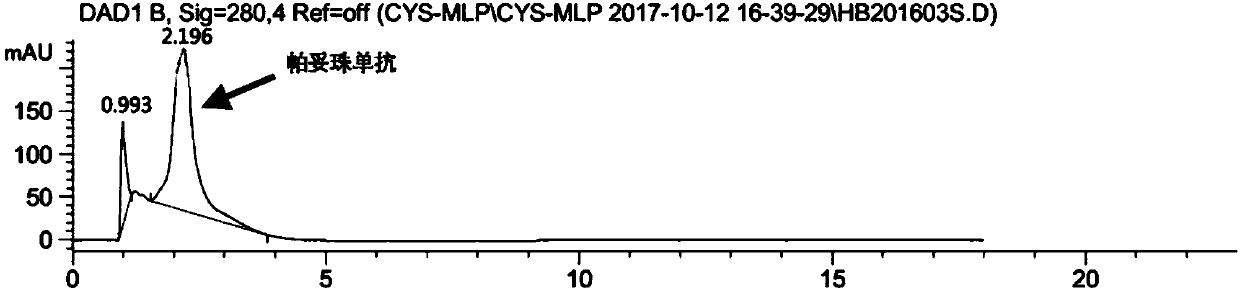
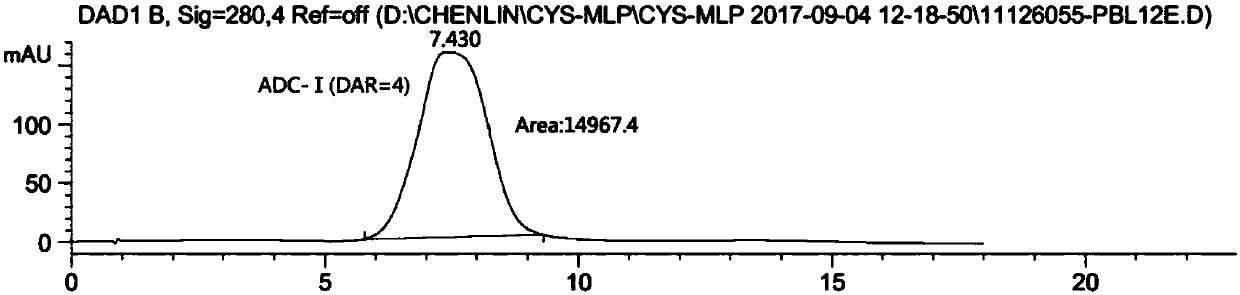
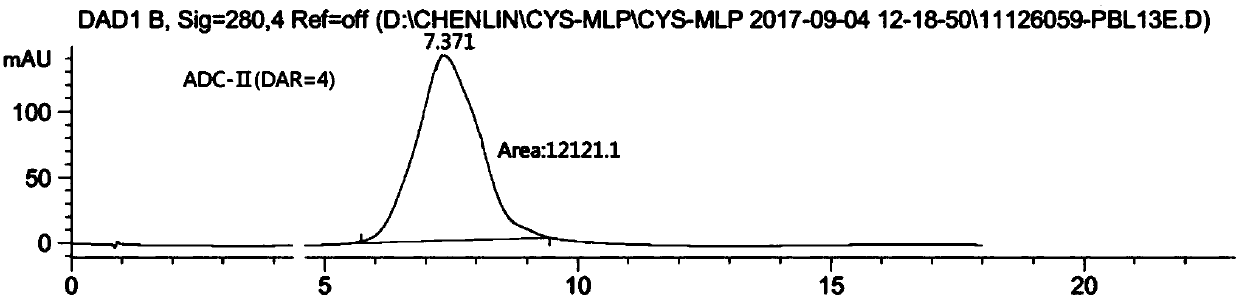
Double-substituted maleic amide connexon for antibody-medicine coupling as well as preparation method and application of connexon
A technology of maleamide and drug conjugates, which is applied in antineoplastic drugs, drug combinations, and pharmaceutical formulations, and can solve unsolved problems such as sulfhydryl exchange, poor chemical stability of coupling reagents, and side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0223] Below in conjunction with specific embodiment, further illustrate the present invention. It should be understood that these examples are only used to illustrate the present invention and are not intended to limit the scope of the present invention. For the experimental methods without specific conditions indicated in the following examples, the conventional conditions or the conditions suggested by the manufacturer are usually followed. Percentages and parts are by weight unless otherwise indicated.
[0224] Embodiment group one, compound synthesis and preparation method
[0225] 1.1 Synthesis of Compound E-1 (Formula Ia-1)
[0226] 1.1.1 Intermediate A-1 (step a)
[0227]
[0228] Triethylene glycol (92 g, 613 mmol) was dissolved in tBuOH (200 ml). KOtBu (22.91g, 204mmol) was added under ice-cooling and stirred for half an hour. Under argon protection, a solution of tert-butyl bromoacetate (39.8g, 204mmol) in tBuOH (40ml) was added dropwise, and stirred overnigh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com