Antibody-drug conjugate targeting gpc3 and its preparation method and use
A technology of drug conjugates and antibody conjugates, which is applied in the field of medicine, can solve the problems of poor chemical stability of coupling reagents, long synthetic routes of coupling reagents, and messy electrophoretic images of antibody conjugates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0209] Antibody preparation
[0210] The sequence of the DNA molecule of the antibody or fragment thereof of the present invention can be obtained by conventional techniques, such as PCR amplification or genomic library screening. In addition, the coding sequences for the light and heavy chains can be fused together to form single-chain antibodies.
[0211] Once the relevant sequences are obtained, recombinant methods can be used to obtain the relevant sequences in large quantities. Usually, it is cloned into a vector, then transformed into a cell, and then the relevant sequence is isolated from the proliferated host cell by conventional methods.
[0212] In addition, related sequences can also be synthesized by artificial synthesis, especially when the fragment length is relatively short. Often, fragments with very long sequences are obtained by synthesizing multiple small fragments and then ligating them.
[0213] At present, the DNA sequence encoding the antibody of the ...
Embodiment 1
[0389] Synthesis and preparation of the compound shown in embodiment 1, formula Ic
[0390] The substituted maleamide linker fragment molecule represented by formula Ia listed in the first aspect of the present invention can be synthesized by the method in Scheme 1, and intermediate A is obtained by reacting n glycol with tert-butyl bromoacetate, and then combined with Aromatic nucleophilic substitution of substituted nitrofluorobenzenes affords intermediate B. In addition, intermediate B can also be obtained by reacting p-tosylate-protected intermediate F with substituted nitrofluorophenols. The nitro group in intermediate B is reduced to amino to obtain intermediate C, which is then reacted with 2,3-dibromomaleic anhydride to obtain intermediate D, and then subjected to substitution reaction with arylthiophenol to obtain the linker fragment Molecule E. Molecules of series F are obtained by condensation with dipeptide / tripeptide-PAB cytotoxic drug linkers. The reaction sch...
Embodiment 2
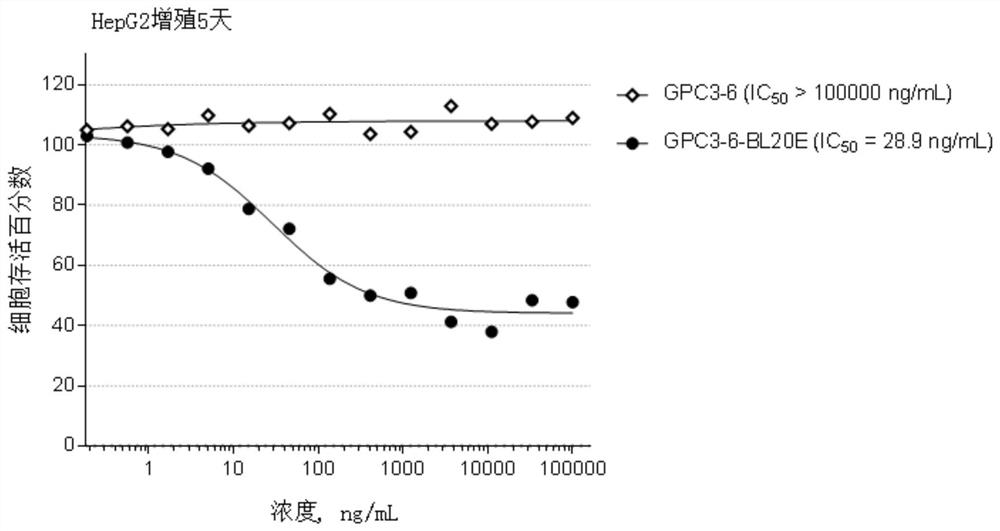
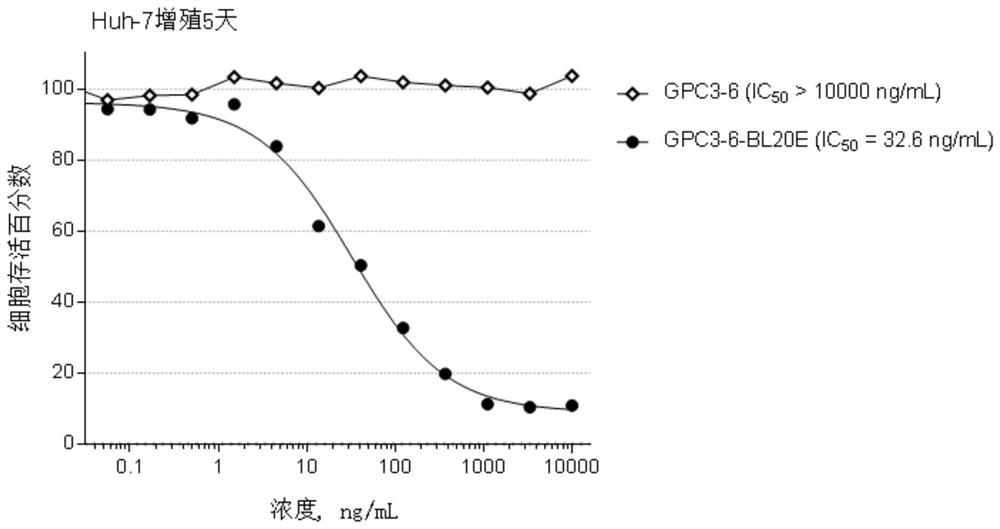
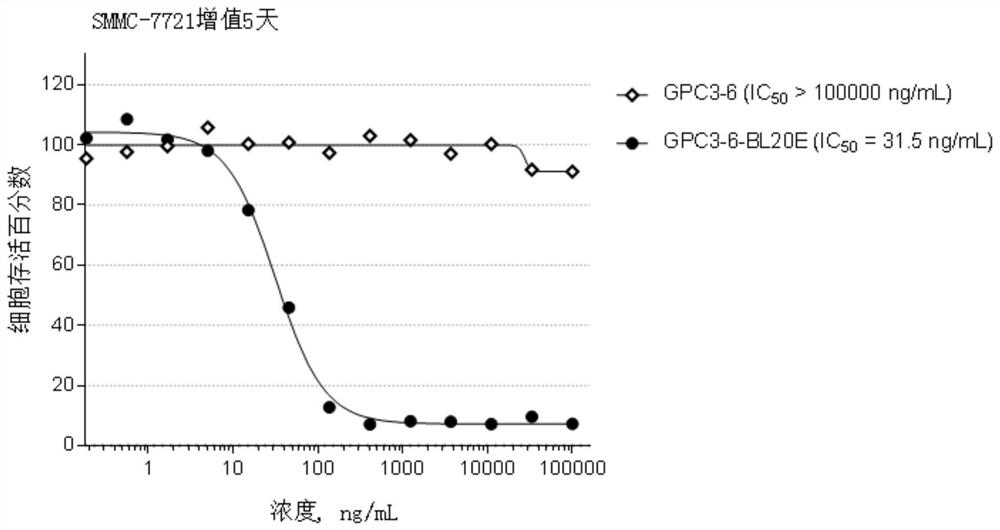
[0435] Example 2, Preparation and identification of anti-GPC3 antibody
[0436] The GPC3 antibody of the present invention is prepared by the following method: use a phage display antibody library to screen against GPC3 to obtain a human anti-GPC3 antibody, and sequence and identify the antibody with good affinity. The sequence information is as follows:
[0437] Table 2 Human anti-GPC3 antibody GPC3-6 heavy chain variable region CDR and light chain variable region CDR
[0438]
[0439] Table 3 Sequences of human anti-GPC3 antibody GPC3-6 heavy chain and light chain framework regions (FR 1-4)
[0440]
[0441]
[0442] Note: In the above table, aa represents the amino acid sequence, and nt represents the nucleotide sequence.
[0443] SEQ ID NO: 30GPC3-6 heavy chain variable region (VH) amino acid sequence 120aa
[0444] QVHLVQSGAEVQKPGSSVKVSCKASGGTFSSYGINWVRQAPGQGLEWMGWISAYNGNTNYAQKLQGRVTMTTDTSTSTAYMELRSLRSDDTA VYYCARGSGLLPRIGGYWGKGTMVTVSS
[0445] SEQ ID NO:29GPC3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com