TF-targeted antibody-drug conjugate and preparation method and application thereof
A technology of drug conjugates and conjugates, applied in the field of medicine, can solve the problems of poor chemical stability of coupling reagents, long synthesis routes of coupling reagents, unresolved sulfhydryl exchange and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0127] Antibody preparation
[0128] The sequence of the DNA molecule of the antibody or fragment thereof of the present invention can be obtained by conventional techniques, such as PCR amplification or genomic library screening. In addition, the coding sequences for the light and heavy chains can be fused together to form single-chain antibodies.
[0129] Once the relevant sequences are obtained, recombinant methods can be used to obtain the relevant sequences in large quantities. Usually, it is cloned into a vector, then transformed into a cell, and then the relevant sequence is isolated from the proliferated host cell by conventional methods.
[0130] In addition, related sequences can also be synthesized by artificial synthesis, especially when the fragment length is relatively short. Often, fragments with very long sequences are obtained by synthesizing multiple small fragments and then ligating them.
[0131] At present, the DNA sequence encoding the antibody of the ...
Embodiment 1
[0233] Synthesis and preparation of the compound shown in embodiment 1, formula Ic
[0234] The substituted maleamide linker fragment molecule represented by formula Ia listed in the first aspect of the present invention can be synthesized by the method in Scheme 1, and intermediate A is obtained by reacting n glycol with tert-butyl bromoacetate, and then combined with Aromatic nucleophilic substitution of substituted nitrofluorobenzenes affords intermediate B. In addition, intermediate B can also be obtained by reacting p-tosylate-protected intermediate F with substituted nitrofluorophenols. The nitro group in intermediate B is reduced to amino to obtain intermediate C, which is then reacted with 2,3-dibromomaleic anhydride to obtain intermediate D, and then subjected to substitution reaction with arylthiophenol to obtain the linker fragment Molecule E. Molecules of series F are obtained by condensation with dipeptide / tripeptide-PAB cytotoxic drug linkers. The reaction sch...
Embodiment 2
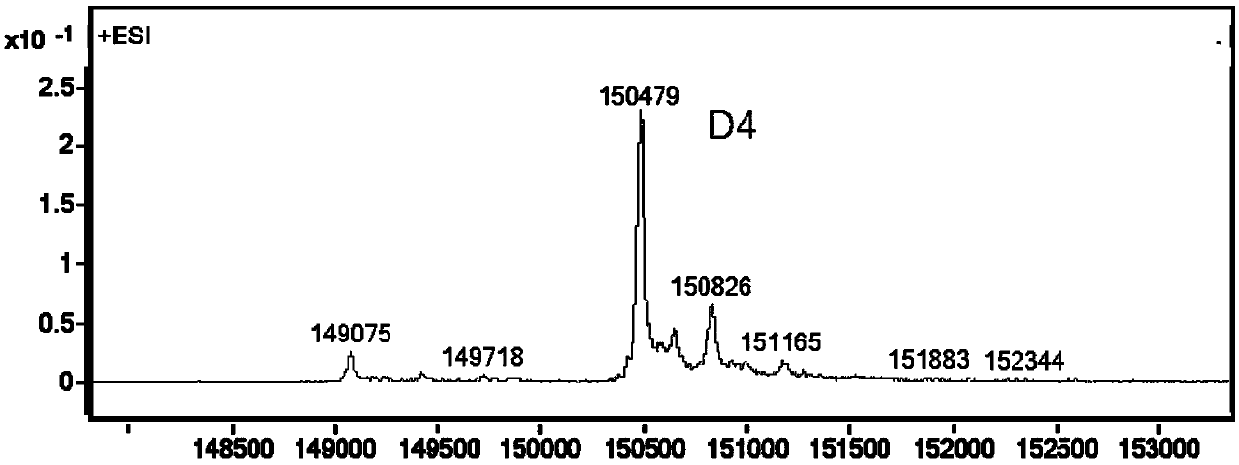
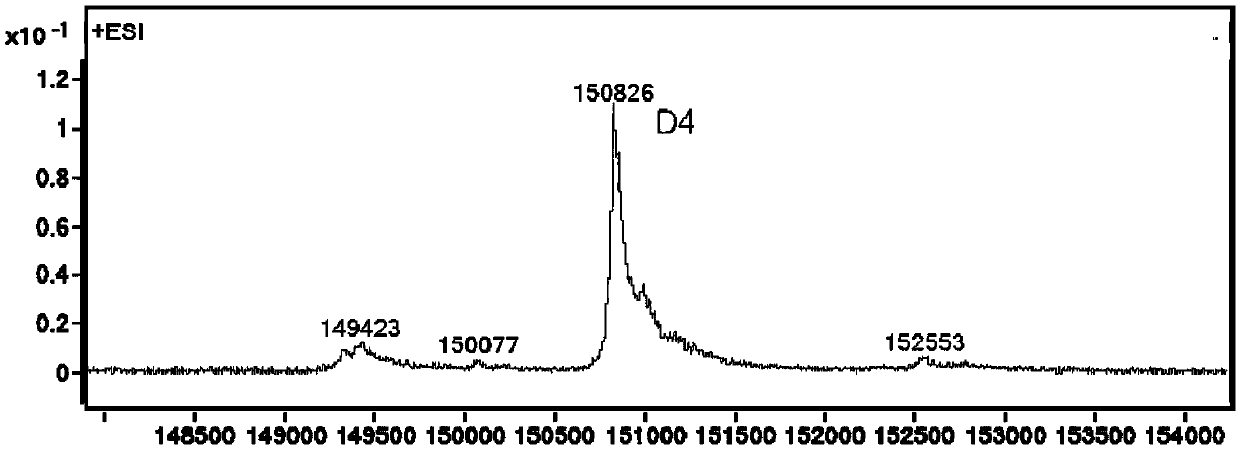
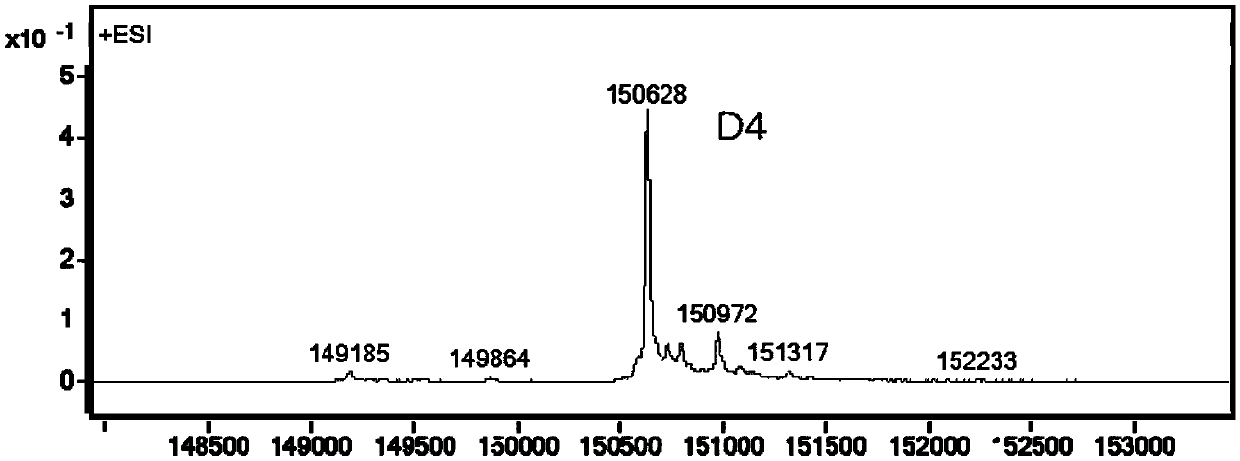
[0280] Embodiment 2, preparation of antibody conjugate
[0281] 2.1 Preparation of TF-mAb-H39-BL9-MMAE
[0282] The stock solution of the humanized antibody TF-mAb-H39 targeting TF was treated with 50 mM potassium dihydrogen phosphate-sodium hydroxide (KH 2 PO 4 -NaOH) / 150mM sodium chloride (NaCl) / 1mM diethyltriaminepentaacetic acid (DTPA), pH7.4 reaction buffer diluted to 5mg / mL, adding 10-fold excess molar ratio of tris(2-carboxyethyl ) Phosphine hydrochloride (TCEP), the reaction solution was stirred at 10°C for 4 hours.
[0283] The above reaction liquid was cooled to 5°C, an appropriate amount of diethylacetamide (DMA) was added without purification, and then compound Ic-1 prepared in Example 1 (10 mg / ml pre-dissolved in DMA Middle), ensure that the volume ratio of DMA in the reaction system does not exceed 10%, and agitate at 25°C for 2.5 hours for coupling.
[0284] The coupling reaction mixture was purified by histidine-acetic acid / sucrose gel filtration at pH 6.0 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com