Disubstituted maleamide linker for antibody-drug conjugation and its preparation method and use
A maleamide and linker technology, applied in the field of new disulfide chain bridging and cross-linking reagents, can solve the problems of high R&D and final industrialization costs, time-consuming large-scale preparation and production, and low antibody/protein expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0140] Preparation method of antibody-drug conjugate
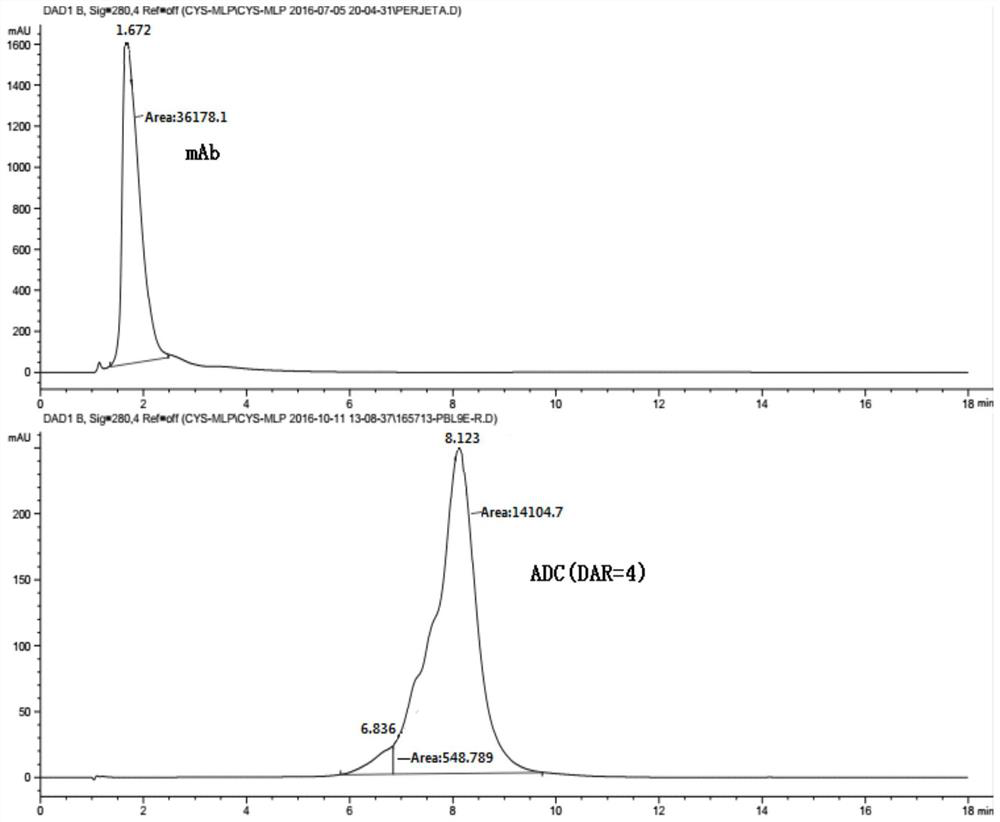
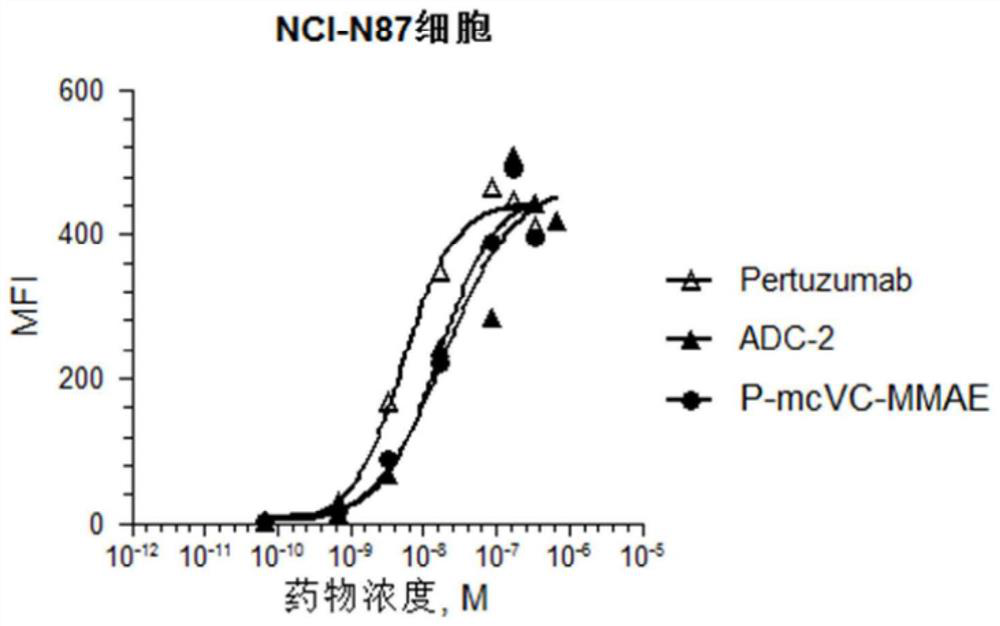
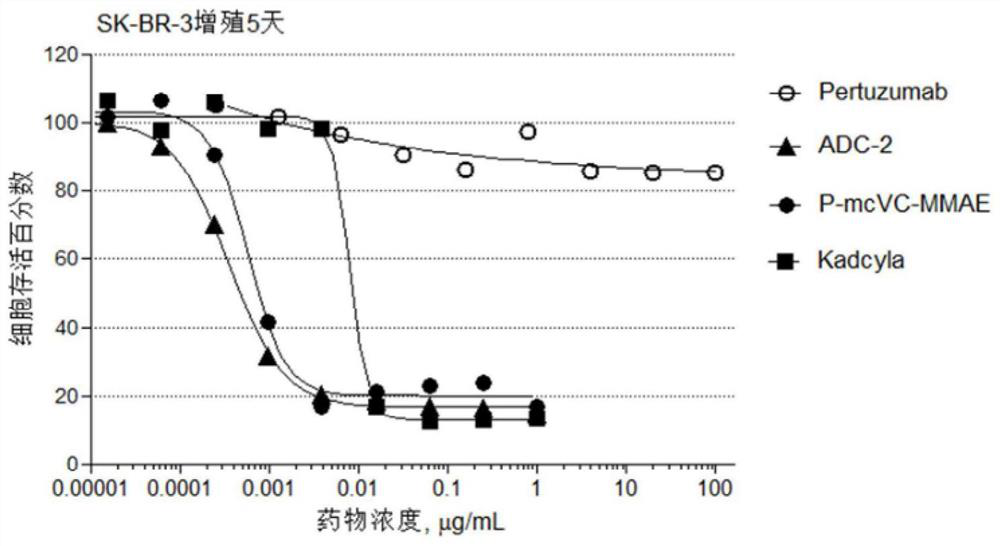
[0141] The antibody-drug conjugate preparation route is shown below. The disulfide bonds between the antibody chains are reduced to generate a total of 8 sulfhydryl groups, and the maleamide linker drug conjugate is cross-linked with the reduced antibody sulfhydryl group to generate the corresponding antibody drug conjugate, wherein the antibody drug The conjugate exists in one or two forms as in the following reactions.
[0142]
[0143] Dilute the antibody stock solution to 2-10mg / mL with reaction buffer, add 140-200 times excess molar ratio of dithiothreitol (DTT), or add 6.0-20 times excess molar ratio of tris(2-carboxyethyl ) phosphine hydrochloride (TCEP), the reaction solution was stirred at 10-35°C for 2-48 hours; the reaction buffer described here can be a buffer prepared in the following ratio: 50mM potassium dihydrogen phosphate-sodium hydroxide (KH 2 PO 4 -NaOH) / 150mM sodium chloride (NaCl) / 1mM diethyltri...
Embodiment 1
[0168] Embodiment 1 (synthesis and preparation of formula 1-12 compound)
[0169] 1.1 Synthesis of Compound F-1 (Formula 1)
[0170] 1.1.1 Synthesis of intermediate B-1 (step a)
[0171]
[0172] Weigh 4-fluoronitrobenzene (10.0g, 0.071mol), diethylene glycol (75.2g, 0.71mol) and potassium carbonate (14.7g, 0.11mol) into a 250mL round bottom bottle, stir at 80°C under nitrogen protection 22 hours. Slowly cool down to room temperature, extract with dichloromethane, wash with 1mol / L dilute hydrochloric acid, water and saturated brine in sequence, dry over anhydrous sodium sulfate, and spin to dry the solvent. Column chromatography (silica gel, 200-300 mesh, PE / EtOAC 10:1) gave the product as a light yellow transparent liquid (15.1 g, yield 94%). LC-MS (M + ) Theoretical value: 227.08, LC-MS (ESI, M+H + ) measured value: 228.12.
[0173] 1.1.2 Synthesis of intermediate C-1 (step b)
[0174]
[0175] Intermediate compound B-1 (3.0g, 9.52mmol) was dissolved in acetone ...
Embodiment 2
[0241] Embodiment 2 (synthesis and preparation of formula 19-25 compound)
[0242] 2.1 Synthesis of Compound G-1 (Formula 19)
[0243]
[0244] Compound F-3 (200mg, 0.34mmol) and compound D1 (220mg, 0.34mmol) were dissolved in N,N-dimethylformamide (10 mL), and 1-ethyl-(3-dimethyl Aminopropyl) carbodiimide hydrochloride (EDCI) (77 mg, 0.40 mmol) and 1-hydroxybenzotriazole (HOBT) (54 mg, 0.40 mmol), and the reaction mixture was stirred at room temperature overnight. The reaction solution was diluted with ethyl acetate, extracted with water, washed with water and saturated brine successively, dried over anhydrous sodium sulfate, and spun to dry the solvent. The crude product was separated and purified by silica gel chromatography (dichloromethane / methanol), and dried by suction to obtain yellow amorphous solid G-1 (3.5 g, yield 98%). LC-MS (M + ) Theoretical value: 1226.40, LC-MS (ESI, M+H + ) measured value: 1227.42.
[0245] 2.2 Synthesis of Compound G-2 (Formula 20) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com