Dactylicapnos scandens derivative, pharmaceutical composition thereof, preparation method and application
A compound and pharmaceutical technology, which is applied in the field of preparation of drugs for treating pain, can solve problems such as the structure and medicinal activity of compounds that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
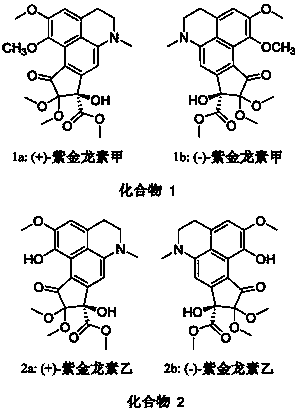
[0051] Separation and purification of compounds 1 and 2:
[0052] Take 3.9 kg of air-dried Zijinlong plant roots, grind them, heat them with 80% ethanol at 70°C, and extract them under reflux for 3 times, recover the solvent, concentrate to a small volume, add 1% HCl to adjust the pH value to 2, and extract 3 times with ethyl acetate. Once, the aqueous layer was adjusted to pH 9 with 10% ammonia water, then extracted 3 times with ethyl acetate, the ethyl acetate layer (104g) was concentrated, and after the ethyl acetate part was mixed with silica gel, 700g, 200-300 mesh silica gel was carried out. Column chromatography, with chloroform / methanol gradient elution segmented, the distribution ratio is chloroform / methanol 10:0, 20:1, 10:1, 8:1, 5:1, 3:1, 1:1, according to each 300 mL portions were pooled into 7 pooled Frs 1-7. Component Fr3 uses methanol / water 35:65, 50:50, 60:40, 70:30, 80:20, 90:10, 100:0 as the eluent, TLC detects the same component of the merged spot to obtain...
Embodiment 2
[0080] The four compounds in Example 1 can all activate TRPV1 ion channels, and may have better analgesic effects than the positive control drug capsaincin. The experimental methods and results are as follows:
[0081] 1. Materials and methods:
[0082] 1.1 Human embryonic kidney cell (HEK293) cell culture and transfection
[0084] When the cells are thawed, the frozen cells should be thawed quickly, and the frozen tube can be shaken from time to time to make it pass through the most vulnerable temperature range (-5-0°C) as soon as possible to ensure high cell survival rate, good growth and shape. Specific steps are as follows:
[0085] ①Take out the cells from the liquid nitrogen tank, put them into warm water at 37°C to thaw quickly and stir constantly, so that the cells in the cryopreservation tube can be thawed as soon as possible (within one minute), and then take out the cells under sterile conditions.
[0086] ② After aseptically removing ...
Embodiment 3
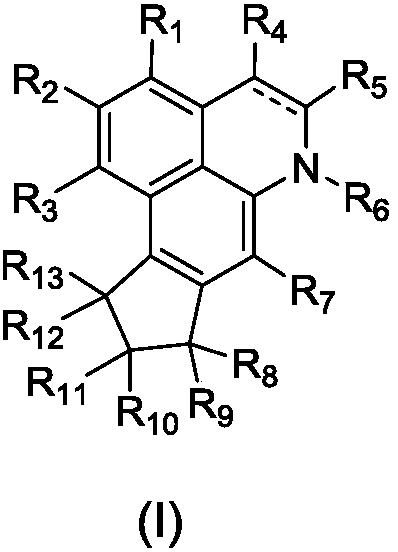
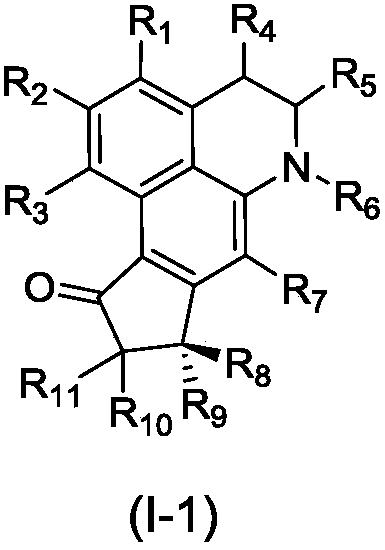
[0114] Examples of structural modification and modification of compounds 1 and 2:
[0115]
[0116] Tested by the method of Example 2, the modified compound 3-6 has similar activity to compounds 1 and 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com