Application of Regorafenib hydrate in preparing drug for treating pulmonary fibrosis
A technology for pulmonary fibrosis and regorafenib, which is applied in the application field of regorafenib hydrate in the preparation of drugs for treating pulmonary fibrosis diseases, and achieves good application prospects, no adverse reactions, and effects of slowing pulmonary fibrosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
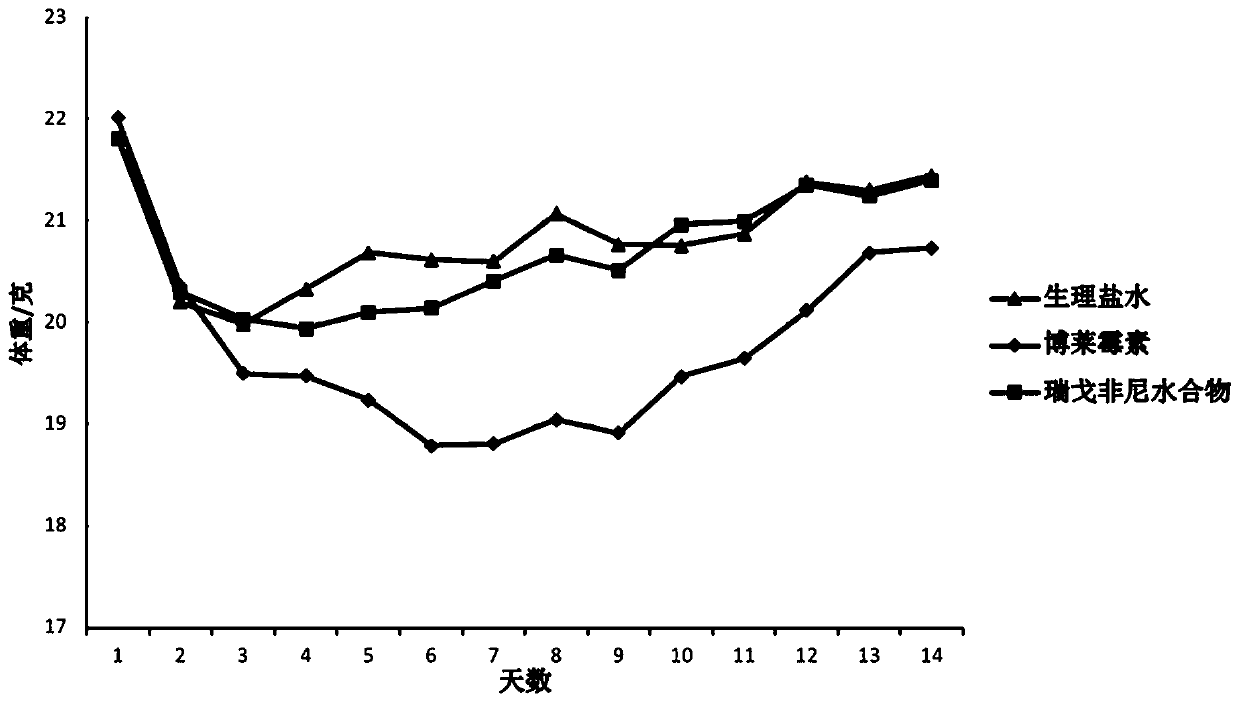
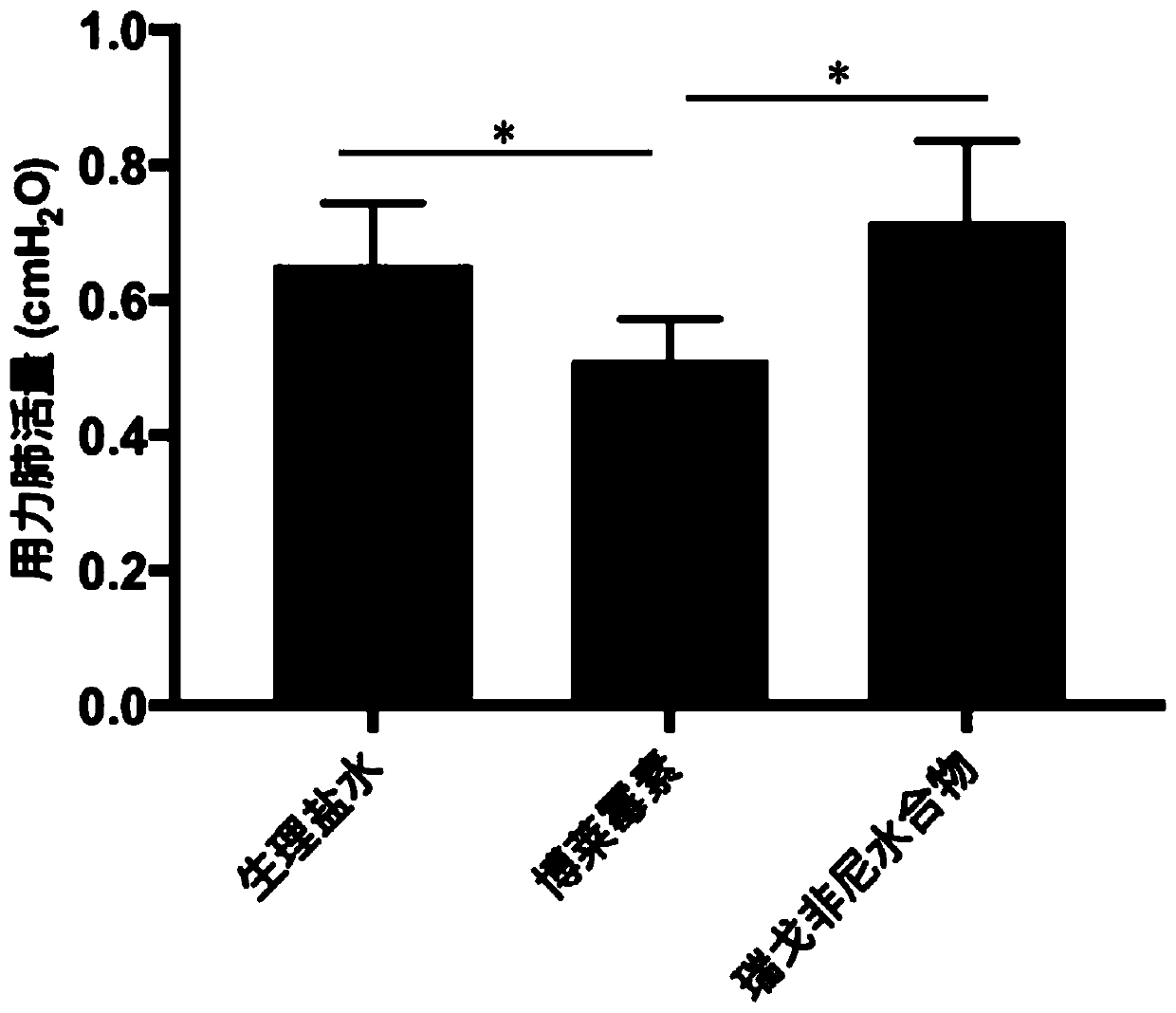
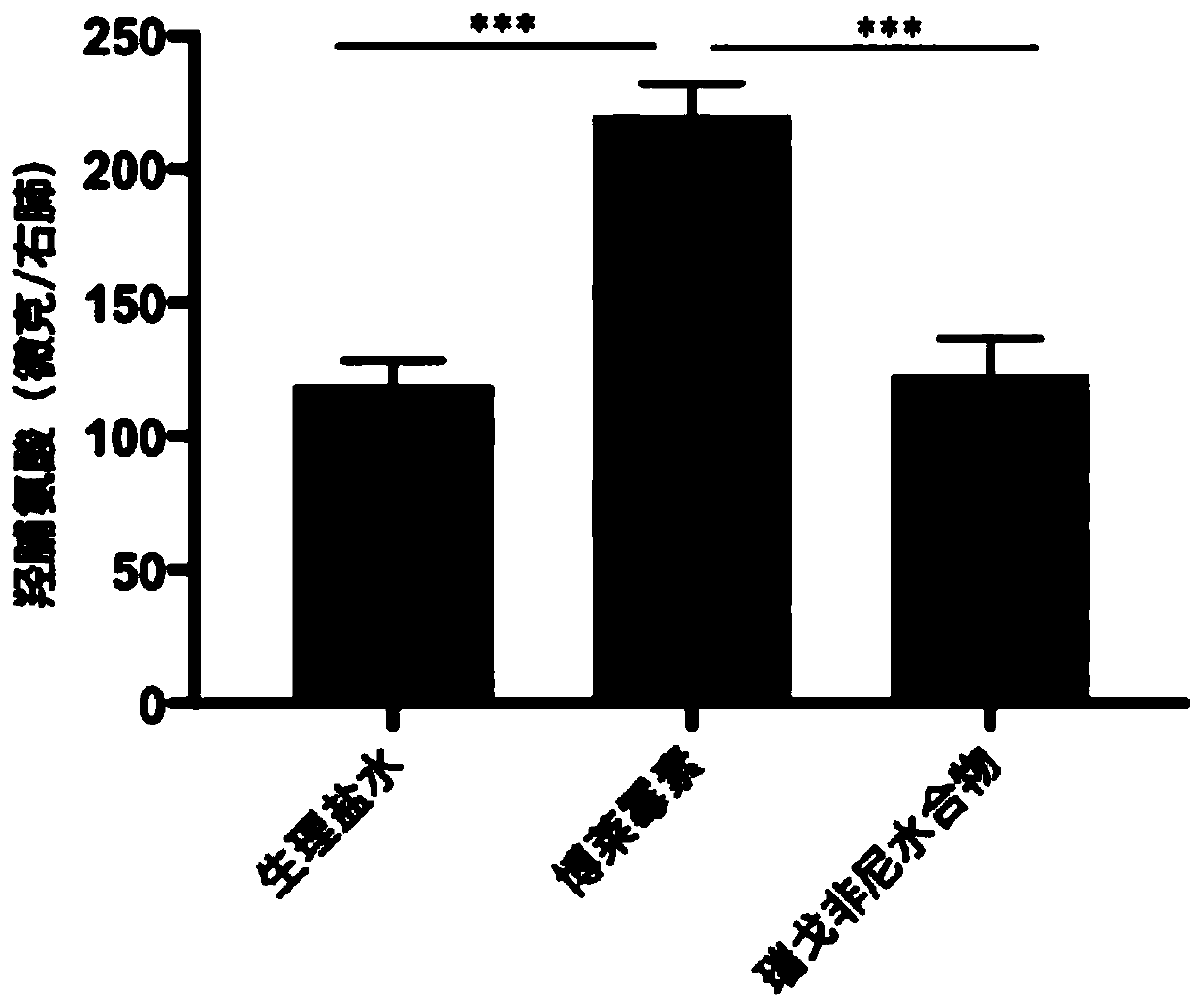
[0022] Example: Regorafenib hydrate slows down bleomycin-induced lung fibrosis in mice.
[0023] Animal model preparation: male C57BL / 6J, wild-type mice (8-10 weeks old), give mice anesthesia by intraperitoneal injection of 0.5ml / 100g (body weight) with mass volume solubility 10% chloral hydrate, and there is Intravenous injection of 2U / Kg bleomycin.
specific Embodiment approach
[0024] The specific implementation method is as follows: after the mouse is anesthetized, weigh and record, fix the mouse on the operating table, disinfect the neck with 70% alcohol, use a scalpel to make a vertical wound of about 1 cm in the neck of the mouse, and use micro tweezers to separate The tissue exposed the trachea, and the syringe was pierced into the trachea through the gap between the tracheal cartilage rings toward the heart, and then slowly injected with a volume of bleomycin saline solution corresponding to its body weight at a rate of 2 U / kg, and immediately turned the animal upright and left and right to make The liquid medicine is evenly distributed in the lungs.
[0025] The blank control group was injected with the same volume of normal saline (0.9% Nacl).
[0026] Group administration: Regorafenib hydrate treatment refers to giving mice 30 mg / kg regorafenib hydrate by intragastric administration every day on the 7th to 14th day of bleomycin treatment, wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com