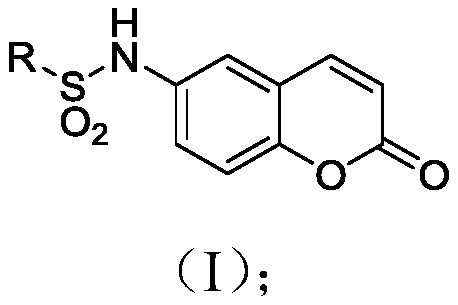
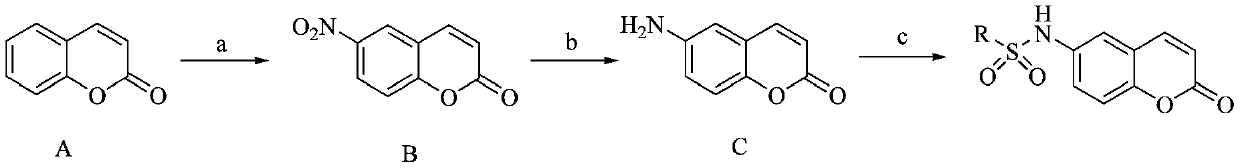
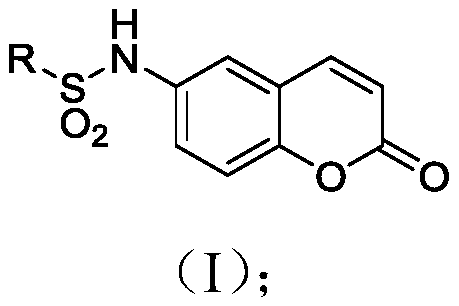
Coumarin compound and drug combination and preparation and application methods thereof
A technology of coumarins and compounds, which is applied in the direction of drug combinations, active ingredients of heterocyclic compounds, pharmaceutical formulations, etc., can solve the problem of limiting the anti-tumor potential of Bromodomain protein, the small molecule inhibitor structure type of BRD4 protein, The effect still needs to be improved to achieve significant protein binding ability, good anti-tumor activity, and good protein inhibitory activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Synthesis of 6-nitro-2H-benzopyran-2-one (II):
[0055] Add HNO to a 250mL three-necked bottle 3 (1.3g, 20.1mmol) and H 2 SO 4 10mL, placed in an ice-salt bath and stirred, maintaining the temperature at 0°C, adding coumarin (2.9g, 20mmol) in H 2 SO 4 2mL, and then maintain the temperature at 0°C for about 2h. TLC detected that the reaction was complete. Pour the reaction liquid into 300mL ice-water mixture and stir while adding. After the ice melted, a solid precipitated out. Suction filtration and vacuum drying of the filter cake gave a yellow solid , yield 64.8%.
[0056] (2) 6-amino-2H-benzopyran-2-one (III):
[0057] Add iron powder (1.7g, 31mmol) and NH 4 Cl (0.8g, 15mmol), then add ethanol (30mL), water (10mL), add 6-nitro-2H-chromen-2-one (II) (1.5g, 7.8mmol) to the above system , reacted at 80°C for 2 hours, filtered with suction, distilled off the solvent by adding pressure, and separated by column chromatography to obtain 6-amino-2H-benzopyran-2-...
Embodiment 2
[0063] Synthesis of N-(2-oxo-2H-benzopyran-6-yl)cyclohexylsulfonamide (2): The specific preparation method is the same as in Example 1, and the yield obtained is 92.3%.
[0064] 1 H NMR (400MHz, DMSO) δ9.97(s, 1H), 8.09(d, J=9.6Hz, 1H), 7.56(d, J=2.4Hz, 1H), 7.45(dd, J=8.9, 2.5Hz ,1H),7.39(d,J=8.9Hz,1H),6.50(d,J=9.6Hz,1H),3.03(t,J=10.2Hz,1H),2.05(d,J=11.5Hz,2H ),1.76(d,J=12.9Hz,2H),1.59(d,J=12.0Hz,1H),1.43(m,2H),1.18(m,3H). 13 C NMR (101MHz, DMSO) δ160.31, 150.34, 144.55, 135.38, 124.39, 119.63, 118.65, 117.74, 117.23, 59.50, 26.45, 25.19, 24.77. HRMS (ESI) calcd.forC 15 h 18 NO 4 S([M+H] + )308.0957, found 308.0952.
Embodiment 3
[0066] Synthesis of N-(2-oxo-2H-benzopyran-6-yl)cyclopentasulfonamide (3): The specific preparation method is the same as in Example 1, and the yield obtained is 85.9%.
[0067] 1 H NMR (400MHz, DMSO) δ9.95 (s, 1H), 8.10 (d, J = 9.6Hz, 1H), 7.57 (d, J = 2.3Hz, 1H), 7.48–7.37 (m, 2H), 6.50 (d,J=9.7Hz,1H),3.64–3.49(m,1H),1.94–1.85(m,4H),1.65(m,4H),1.55(m,4H). 13 C NMR (101MHz, DMSO) δ160.31, 150.53, 144.52, 135.20, 124.93, 119.63, 119.25, 117.73, 117.23, 60.21, 27.77, 25.87. HRMS (ESI) calcd.for C 14 h 16 NO 4 S([M+H] + )294.0800, found 294.0794.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com