A simple method for the synthesis of ubiquitin probe ub-rho110-gly
A ubiquitin and probe technology, applied in the field of protein synthesis, can solve problems such as high toxicity and limitation, and achieve the effects of low synthesis cost, simple synthesis steps and high preparation yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The ubiquitin mutant Ub77C monoclonal colonies were picked and placed in 40 mL of LB medium containing ampicillin resistance, and cultured at 37° C. and 220 rpm for 14 h.
[0036] Take the bacterial liquid cultured in the above steps and expand it into 4L LB medium containing ampicillin resistance at a volume ratio of 1:100, continue to cultivate at 37 ° C and 220 rpm, and add 1.0 M IPTG when the OD 600 absorbance value of the bacterial liquid reaches 0.8. The final concentration of IPTG in the stock solution was 0.4 mM, the bacterial solution was induced, and the culture was continued for 6 h at 37°C and 200 rpm.
[0037] The cultured bacterial liquid was collected by centrifugation (25°C, 8000 rpm, 10 min), the supernatant was discarded, and the resulting bacterial cells were fully resuspended with 80 mL of lysis buffer (50 mM Tris-HCl, 150 mM NaCl, pH=7.4), and the bacteria were lysed using a sonicator. body. Use a high-speed refrigerated centrifuge to centrifuge th...
Embodiment 2
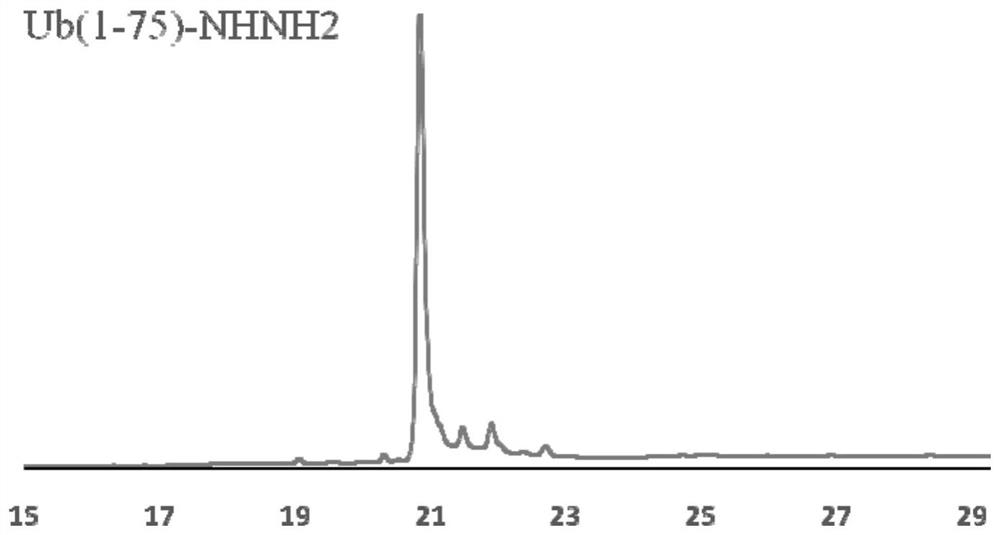
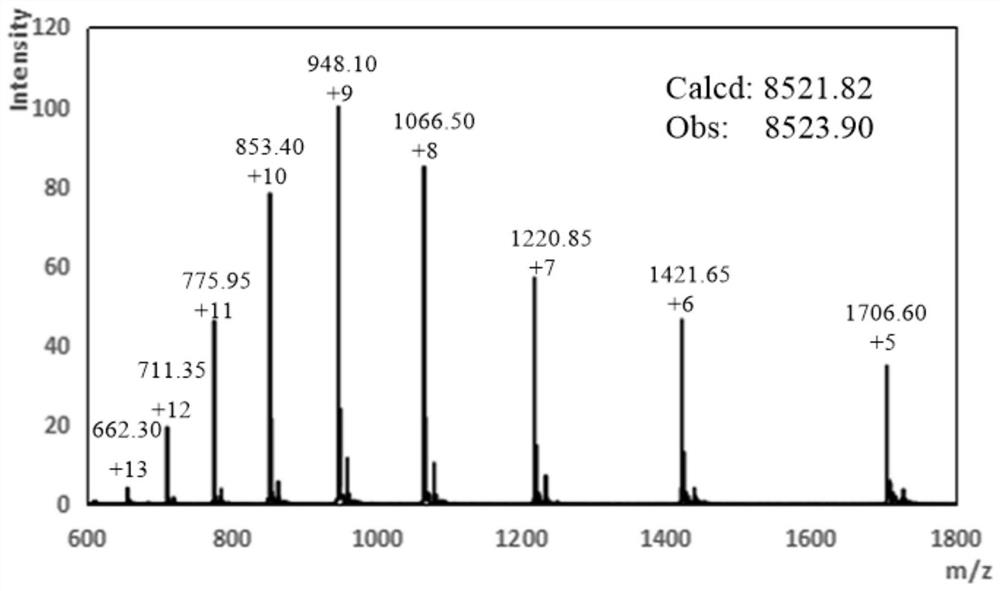
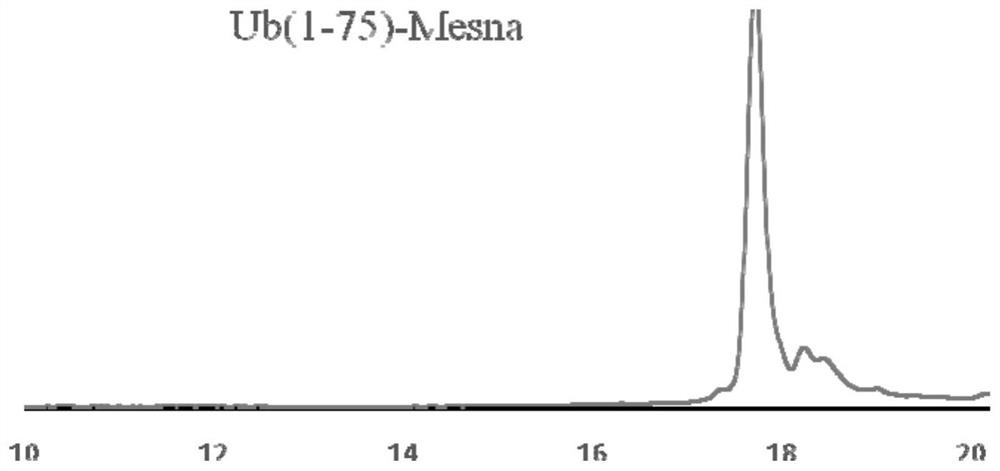
[0041] 50 mg of Ub(1-75)-NHNH 2 Dissolve in 6mL pH=3.0 6M guanidine hydrochloride, 0.2M disodium hydrogen phosphate PBS buffer, add 60uL 1.0M sodium nitrite aqueous solution, react at -20 ℃ for 20min; after the reaction, add 39mg Sodium, and adjust the pH to 5.0, and react at room temperature for 20min; after the reaction is completed, use semi-preparative high performance liquid chromatography to purify the above reaction solution, collect the purified solution, and freeze-dried to obtain 40mg of ubiquitin thioester Ub( 1-75)-Mesna.
Embodiment 3
[0043] Weigh 50 mg of ubiquitin thioester Ub(1-75)-Mesna protein dry powder and dissolve it in 5 mL of pure DMSO, add 2% (volume fraction) thiophenol according to the solution volume, add 129 mg of Gly-Rho110-Gly, and Add 96 mL of DIEA, stir to dissolve, and react at room temperature for 2 h. After the reaction is completed, the reaction solution is precipitated with 20 mL of ether, purified by semi-preparative high performance liquid chromatography, and the purified solution is collected. and freeze-dried to obtain the target product ubiquitin probe Ub-Rho110-Gly.
[0044] In conclusion, the present invention provides a synthetic method for easily obtaining the ubiquitin probe Ub-Rho110-Gly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com