Method for preparing monodisperse core-shell nano catalyst for fuel cell
A core-shell nanotechnology, fuel cell technology, applied in nanotechnology for materials and surface science, battery electrodes, nanotechnology, etc., to ensure the stability of structure and performance, avoid cumbersome operation steps, and facilitate mass production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Add 25mg of sodium borohydride to 20mL of ethylene glycol solution, add 1.2mL of 0.1M cobalt chloride in ethylene glycol solution under vigorous stirring, and stir for 20min.
[0035] 2. Add a mixed solution of 0.4 mL of 0.1 M cobalt chloride and 0.4 mL of 50 mM potassium chloroplatinate dropwise to the above solution, and react for 10 min.
[0036] 3. Add 16mg of XC72 activated carbon to the above solution, stir at 60°C for 6 hours, centrifuge, wash, and dry. The obtained catalyst is denoted as Co 6 @Co 2 Pt 1 / C.
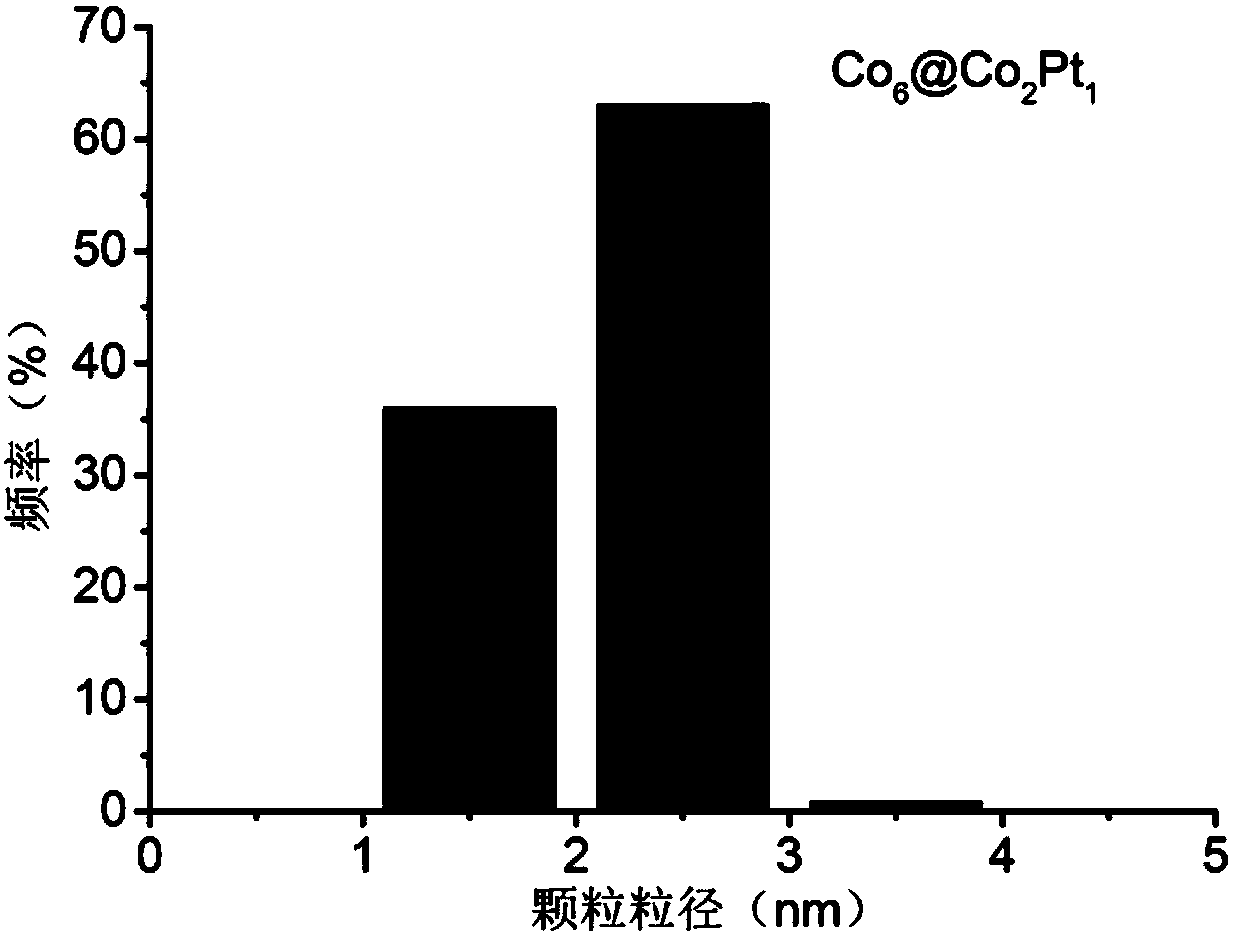
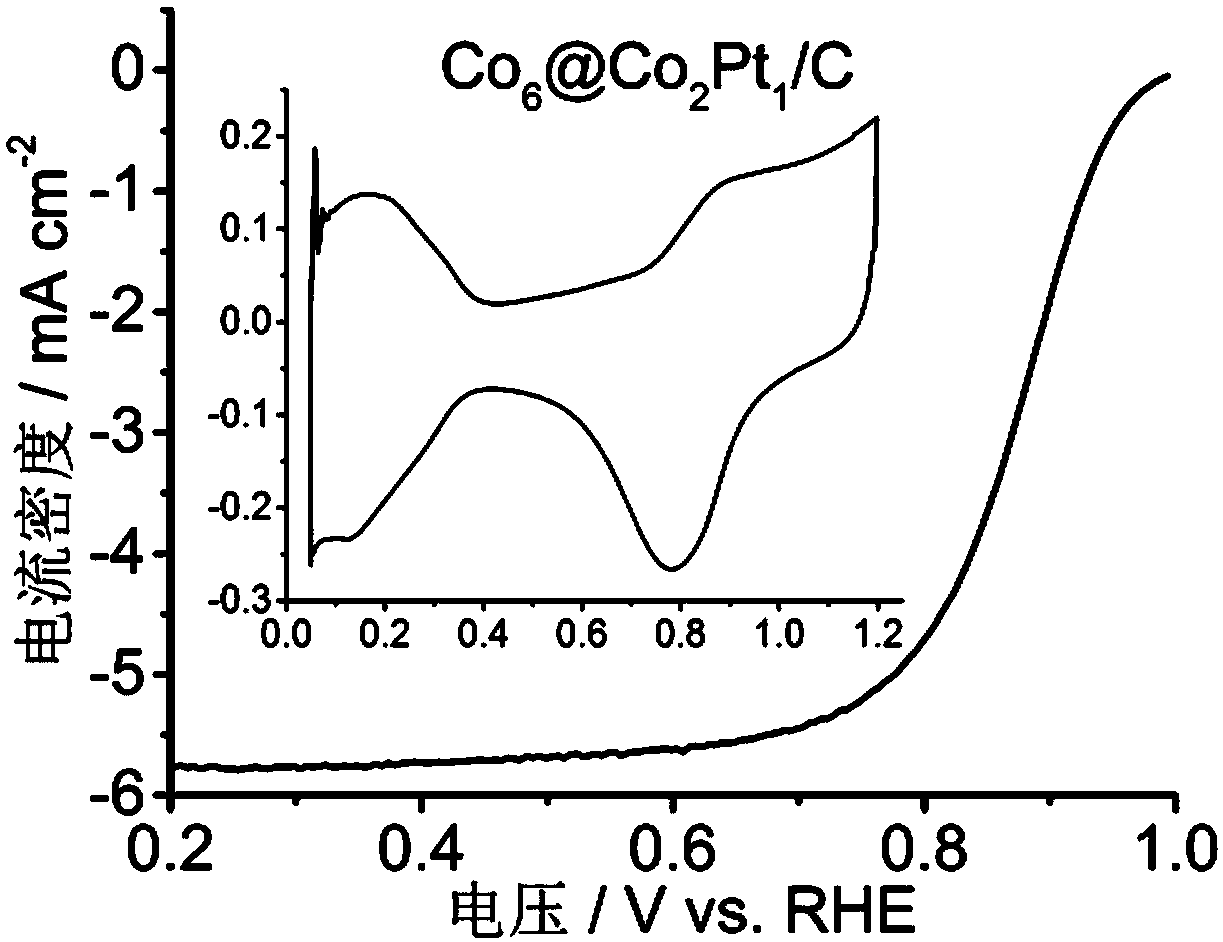
[0037] figure 1 for Co 6 @Co 2 Pt 1 TEM image of / C. figure 2 for Co 6 @Co 2 Pt 1 Size distribution plot of nanoparticles. image 3 for Co 6 @Co 2 Pt 1 Cyclic voltammetry and oxygen reduction polarization curves of / C in rotating disk electrode (RDE) test.
Embodiment 2
[0047] 1. Add 25mg of sodium borohydride into 20mL of ethylene glycol solution, add 1.2mL of 0.1M cobalt chloride in ethylene glycol solution under vigorous stirring, and stir for 30min.
[0048] 2. Add a mixed solution of 0.4mL 0.1M cobalt chloride and 0.4mL 50mM potassium chloroplatinite dropwise to the above solution, and react for 30min.
[0049] 3. Add 16 mg of XC72 activated carbon to the above solution, stir at 90°C for 10 hours, centrifuge, wash, and dry. The obtained catalyst is denoted as Co 6 @Pt 1 / C.
[0050] Figure 6 for Co 6 @Pt 1 TEM image of / C. Figure 7 for Co 6 @Pt 1 Size distribution plot of nanoparticles. Figure 8 for Co 6 @Pt 1 Cyclic voltammetry and oxygen reduction polarization curves of / C in rotating disk electrode (RDE) test.
Embodiment 3
[0052] 1. Add 32mg of sodium borohydride into 20mL of ethylene glycol solution, add 2.0mL of 0.1M cobalt chloride in ethylene glycol solution under vigorous stirring, and stir for 10min.
[0053] 2. Add a mixed solution of 0.2mL 0.1M nickel chloride and 0.4mL 50mM chloroplatinic acid dropwise to the above solution, and react for 10min.
[0054] 3. Add 16 mg of XC72 activated carbon to the above solution, stir at 80°C for 5 hours, centrifuge, wash, and dry. The obtained catalyst is denoted as Co 10 @Ni 1 Pt 1 / C.
[0055] Figure 9 for Co 10 @Ni 1 Pt 1 TEM image of / C. Figure 10 for Co 10 @Ni 1 Pt 1 Size distribution diagram of / C nanoparticles. Figure 11 for Co 10 @Ni 1 Pt 1 Cyclic voltammetry and oxygen reduction polarization curves of / C in rotating disk electrode (RDE) test.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com