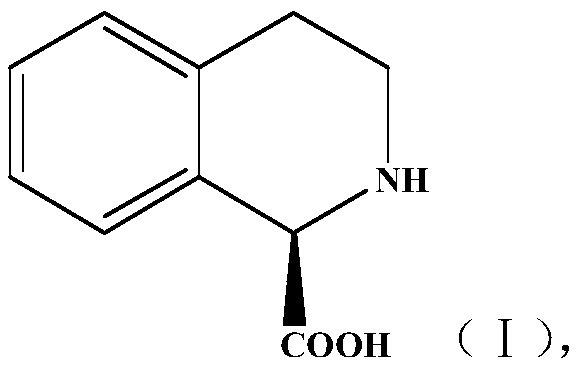
Method for preparing chiral isoquinoline carboxylic acid
A technology of isoquinoline carboxylic acid and isoquinoline carboxylic acid ester, which is applied in the field of preparation of chiral isoquinoline carboxylic acid, can solve problems that have not been seen, and achieve the effects of high production rate, good selectivity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation and separation of embodiment 1 (R)-1-TIC
[0025] Preparation of (R)-1-TIC:
[0026] Preparation of substrate solution: 10 g / L racemate solution of 1-TIC ester was prepared with 0.1 M aqueous ammonium acetate buffer (pH=8.0) and the initial pH value of the solution was adjusted to 8.0 with 30% ammonia water.
[0027] Transfer 300mL of the prepared substrate solution to a round-bottomed flask, set an external constant temperature heating water bath, add QLlip-9 to the substrate solution until the content of QLlip-9 is 5g / L, and react at 30°C for 6h. During the process, the pH value of the reaction solution was always adjusted to be about 8.0 by adding 30% ammonia water. At the end of the reaction, take a certain amount of hydrochloric acid to adjust the reaction solution to be strongly acidic to terminate the reaction, transfer the entire reaction solution to a volumetric flask, use the mobile phase to constant volume, dilute a certain number of times, then ...
Embodiment 2
[0030] Preparation and separation of embodiment 2 (R)-1-TIC
[0031] Preparation of (R)-1-TIC:
[0032] Preparation of substrate solution: 10 g / L racemate solution of 1-TIC ester was prepared with 0.1 M aqueous ammonium acetate buffer (pH=8.0) and the initial pH value of the solution was adjusted to 8.0 with 30% ammonia water.
[0033] Transfer 1000mL of the prepared substrate solution to the reaction container, set an external constant temperature heating water bath, add QLlip-9 to the substrate solution until the content of QLlip-9 is 5g / L, and react at 30°C for 6h, during the reaction process The pH value of the reaction solution was adjusted to always be about 8.0 by adding 30% ammonia water. At the end of the reaction, take a certain amount of hydrochloric acid to adjust the reaction solution to be strongly acidic to terminate the reaction, transfer the entire reaction solution to a volumetric flask, use the mobile phase to constant volume, dilute a certain number of tim...
Embodiment 3
[0036] Preparation and separation of embodiment 3 (R)-1-TIC
[0037] Preparation of (R)-1-TIC:
[0038]Preparation of substrate solution: 10 g / L racemate solution of 1-TIC ester was prepared with 0.1 M aqueous ammonium acetate buffer (pH=8.0) and the initial pH value of the solution was adjusted to 8.0 with 30% ammonia water.
[0039] Transfer 20,000 mL of the prepared substrate solution to a reaction vessel, stir the reactor with an external insulation jacket, add QLlip-9 to the substrate solution until the content of QLlip-9 is 5 g / L, and react at 30°C for 6 hours, During the reaction, the pH value of the reaction solution was adjusted to be about 8.0 by adding 30% ammonia water. At the end of the reaction, take a certain amount of hydrochloric acid to adjust the reaction solution to be strongly acidic to terminate the reaction, transfer the entire reaction solution to a volumetric flask, use the mobile phase to constant volume, dilute a certain number of times, then filter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com