Cyclopolymer containing carbamido large ring and production method and application thereof
A polymer and urea-based technology, applied in the field of cyclized polymers containing urea-based macrocycles and their preparation, can solve problems such as limiting polymer yields, overcome low polymerization yields, high reaction yields, and achieve achievable The effect of controlled change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention provides a kind of preparation method of the cyclic polymer containing ureido macrocycle, comprising:
[0040]A monomer, a chain transfer agent, an initiator and a solvent are mixed and reacted to obtain a ureido-containing macrocyclic cyclic polymer having a structure of formula (II);
[0041] Wherein, the monomer has a structure shown in formula (I),
[0042]
[0043] where the R 1 is hydrogen or C1-C10 alkyl;
[0044] The R 2 is hydrogen or C1-C10 alkyl;
[0045] The X is a C2-C10 straight-chain alkylene group, a C3-C8 cycloalkyl group, a C3-C30 cycloalkyl-containing alkylene group, a C6-C20 arylene group, a C8-C30 aromatic hydrocarbon group-containing Hydrocarbylene or C2-C20 hydrocarbon containing ether group;
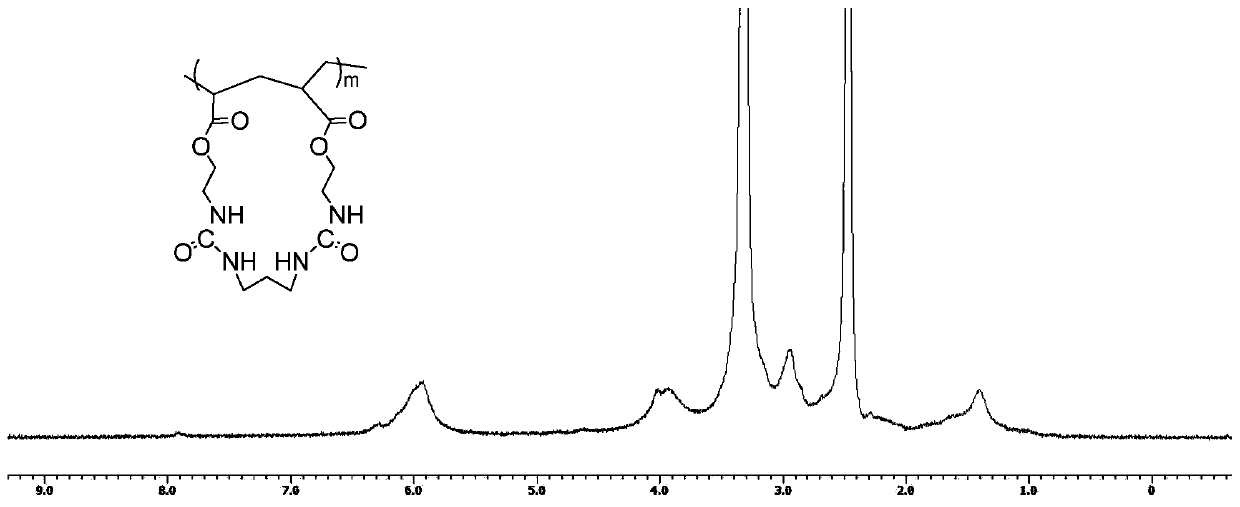
[0046] m is 10-100.
[0047] According to the present invention, the present invention mixes and reacts monomers, chain transfer agents, initiators and solvents to obtain cyclized polymers containing ureido macrocycles; wherei...
Embodiment 1
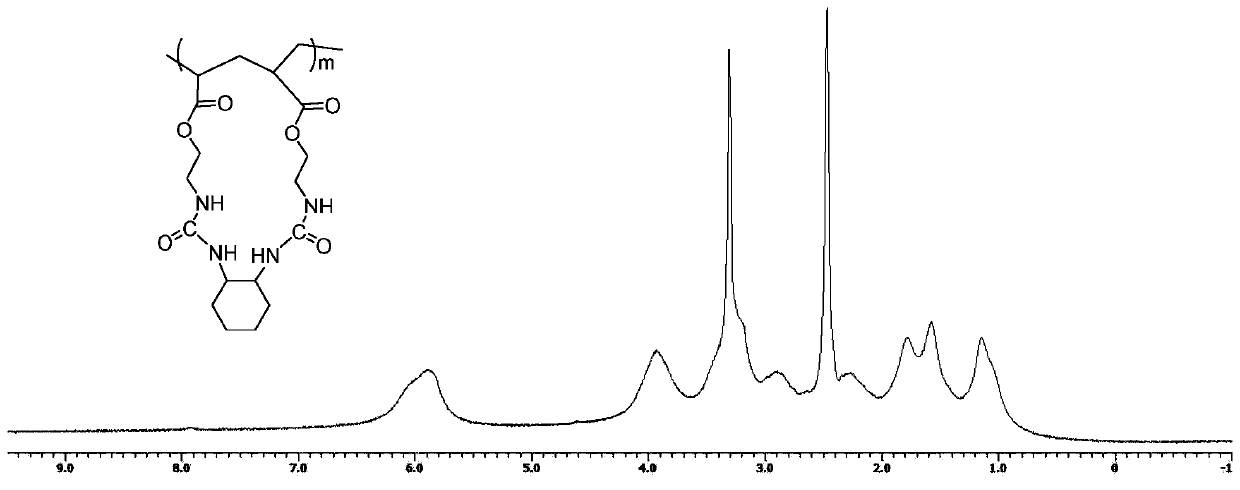
[0081] Monomer: 1,2-bis(acryloyloxyethylurea)cyclohexane (formula I-X-1), in the general formula of bifunctional monomer M, X is ortho-cyclohexyl, R 1 , R 2 for hydrogen. The synthesis steps are as follows:
[0082] Weigh 11.40 g (0.10 mol) of 1,2-cyclohexanediamine, dissolve it in 150 mL of dry dichloromethane, and add acryloyloxyethyl isocyanate (0.20 mol) in dichloromethane dropwise at room temperature under nitrogen protection. Solution 150mL. After the dropwise addition was completed, stirring was continued for 3 hours to obtain a white solid. Suction filtration, washing with dichloromethane three times (30mL×3), and vacuum drying gave 34.10g of 1,2-bis(acryloyloxyethylurea)cyclohexane, m.p.>280°C, yield 86.0%.
[0083] 1 H NMR (400MHz, DMSO, δin ppm): 1.02-1.17 (4H, -CH 2 (CH 2 ) 2 CH 2 -), 1.53-1.55 (2H, -CH 2 (CH2) 2 CH 2 -), 1.79-1.82 (2H, -CH 2 (CH 2 ) 2 CH 2 -), 3.18-3.22 (4H, -COOCH 2 CH 2NH-), 3.95-4.05 (4H, -COOCH 2 CH 2 NH-), 5.81-5.83 (2H, -...
Embodiment 2
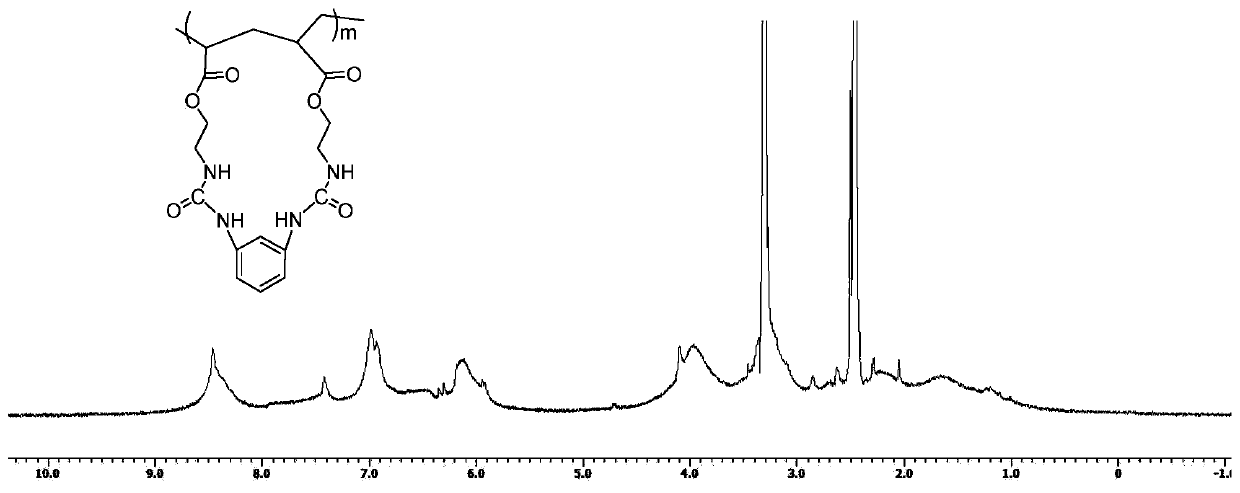
[0086] Monomer: 1,3-bis(acryloyloxyethylurea) benzene (formula I-X-5), in the general formula of bifunctional monomer M, X is meta-phenyl, R 1 , R 2 for hydrogen, R 3 for hydrogen. The synthesis steps are as follows:
[0087] Weigh 10.80 g (0.10 mol) of 1,3-phenylenediamine, dissolve it in 150 mL of dry dichloromethane, and add acryloyloxyethyl isocyanate (0.20 mol) in dichloromethane dropwise at 30°C under nitrogen protection. Solution 150mL. After the dropwise addition was completed, stirring was continued at this temperature for 10 hours to obtain a white solid. Suction filtration, washing with dichloromethane three times (30mL×3), and vacuum drying gave 37.20g of 1,3-bis(acryloyloxyethylurea)benzene, m.p.=180-181°C, yield 95.4%.
[0088] 1 H NMR (400MHz, DMSO, δin ppm): 3.30-3.34 (4H, -COOCH2CH2NH-), 4.09-4.12 (4H, -COOCH2CH2NH-), 5.92-5.94 (2H, -CONHPh), 6.12-6.14 (2H, - CH2NHCO), 6.16-6.35 (6H, CH2=CH-), 6.92-7.02 (2H, Ph), 7.40-7.43 (1H, Ph), 8.48 (1H, Ph).
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com