1h-pyrazol-1-yl-thiazole as an inhibitor of lactate dehydrogenase and methods of using the same
A thiazole, C1-C4 technology, applied in the directions of medical preparations, pharmaceutical formulations, and drug combinations containing active ingredients, can solve problems such as poor bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
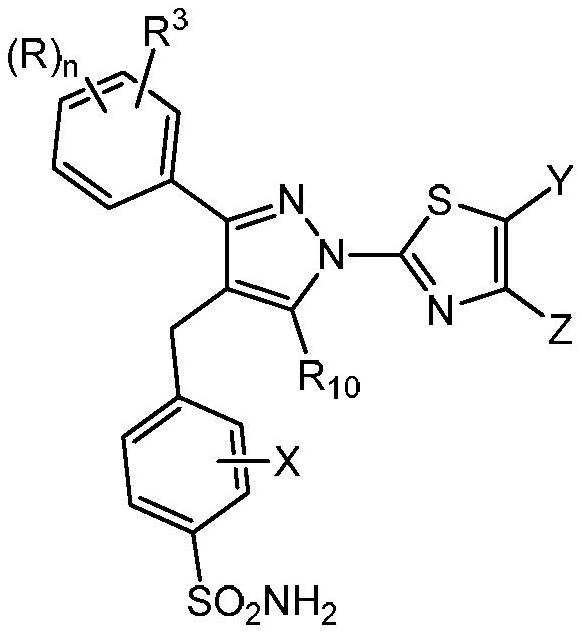
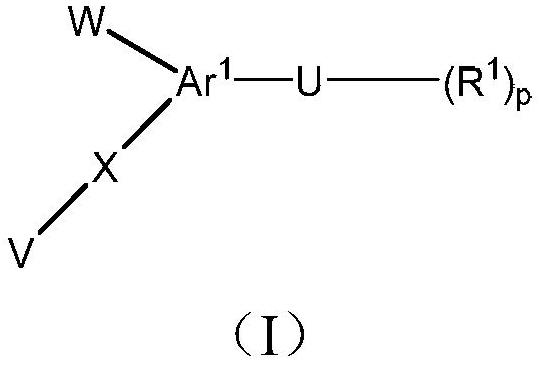
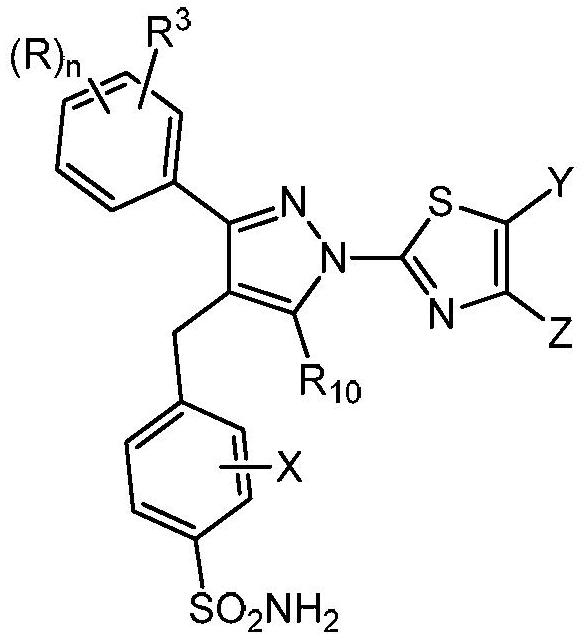
[0113] This example describes a human LDHA primary biochemical assay used to characterize compounds of formula (I) in one embodiment of the present invention.
[0114] Test compounds were placed in a Greiner Bio-One (Monroe, NC) 1536 well black solid bottom assay plate. As assay buffer, 200 millimolar (mM) Tris HCl, pH 7.4, 100 micromolar (μM) EDTA and 0.01% TWEEN-20 were used TM , the final concentration. In assay buffer, the LDHA reagent was 2 nanomolar (nM) human LDHA (Meridian LifeScience, Inc., Memphis, TN), final concentration. In assay buffer, the substrate reagents were 0.06 mM NADH and 0.2 mM sodium pyruvate, final concentrations. In assay buffer, the resazurin / diaphorase coupling agent was 0.037 mM resazurin and 0.133 milligrams per milliliter (mg / mL) diaphorase, final concentrations. The sequence of steps, the number and type of reagents, and the time required for each step are set forth in Table 1. Inhibition of LDHA activity was measured by fluorescence emissi...
Embodiment 2
[0118] This example describes a human LDHB counterscreen biochemical assay used to characterize compounds of formula (I) in one embodiment of the invention.
[0119] Test compounds were placed in a Greiner Bio-One (Monroe, NC) 1536 well black solid bottom assay plate. As assay buffer, use 200 mM Tris HCl, pH 7.4, 100 μM EDTA and 0.01% TWEEN-20 TM , the final concentration. In assay buffer, the LDHB reagent was 2 nM human LDHB (Meridian Life Science, Inc., Memphis, TN), final concentration. In assay buffer, the substrate reagents were 0.13 mM NADH and 0.16 mM sodium pyruvate, final concentrations. In assay buffer, the resazurin / diaphorase coupling agent was 0.037 mM resazurin and 0.133 mg / mL diaphorase, final concentrations. The sequence of steps, the number and type of reagents, and the time required for each step are set forth in Table 2. Inhibition of LDHB activity was measured by fluorescence emission.
[0120] Table 2
[0121]
Embodiment 3
[0123] This example describes a human PHGDH pair screening biochemical assay used to characterize compounds of formula (I) in one embodiment of the present invention.
[0124] Test compounds were placed in a Greiner Bio-One (Monroe, NC) 1536 well black solid bottom assay plate. As assay buffer, 50 mM TEA, pH 8.0, 10 mM MgCl was used 2 , 0.05% BSA, and 0.01% TWEEN-20 TM , the final concentration. In assay buffer, the substrate reagents were 10 μM EDTA, 0.625 mM glutamate, 500 nM human PSAT1, 500 nM human PSPH, 0.05 mM 3-phosphoglycerate, 0.1 mM resazurin, and 0.1 mg / mL diaphorase, final concentration. In assay buffer, PHGDH reagent is 0.15 mM NAD + and 10 nM human PHGDH, final concentration. The sequence of steps, the quantity and type of reagents, and the time required for each step are set forth in Table 3. Inhibition of PHGDH activity was measured by fluorescence emission.
[0125] table 3
[0126]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com