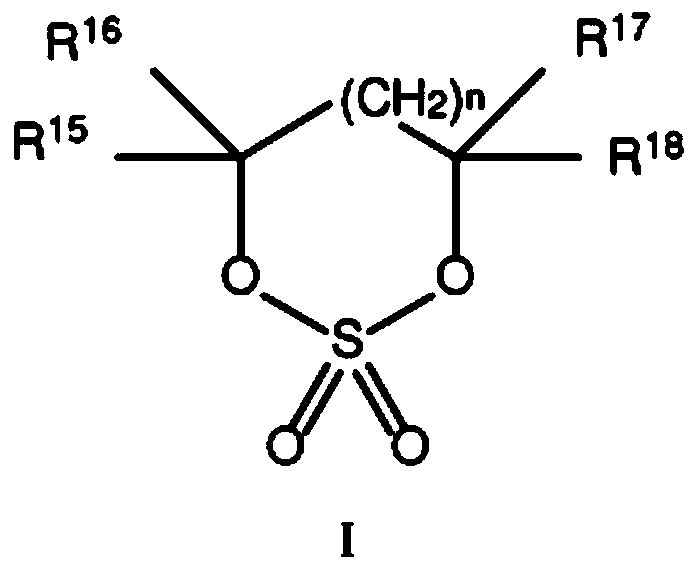
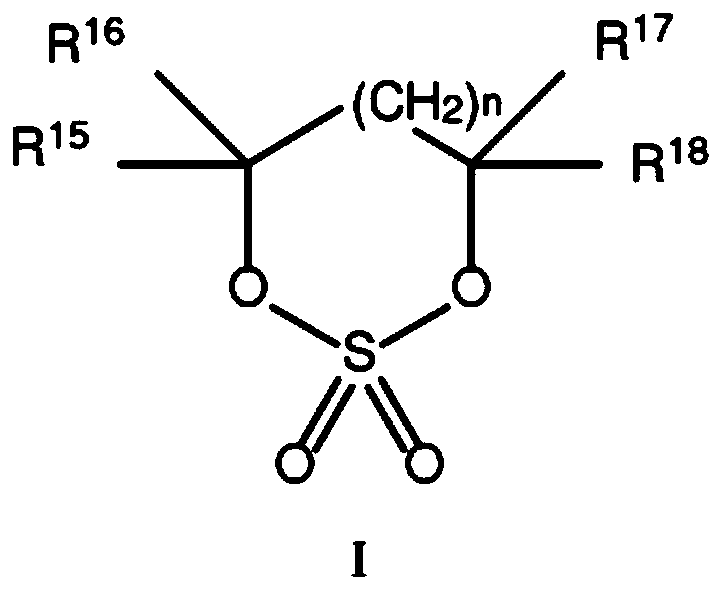
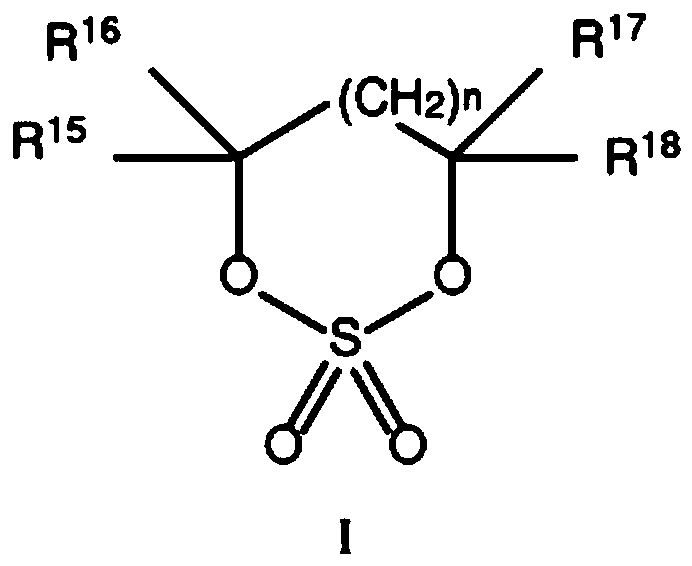
Electrolytes containing six membered ring cyclic sulfates
A cyclic carboxylate, cyclic carbonate technology, applied in electrolytes, circuits, electrical components, etc., can solve the problems of reduced room temperature stability and increased aging of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0190] The following example shows the 1,3 propylene sulfate formulation in 1M LiPF 6 , 75 wt% 2,2-difluoroethyl acetate (hereinafter "DFEA"), 25 wt% fluoroethylene carbonate (FEC) with LiBOB and maleic anhydride (MA). The resulting formulation used in this example contained exactly 1M LiPF 6 With 75wt% DFEA + 25wt% FEC (based on the weight of DFEA and FEC) + 0.85wt% LiBOB + 1.5wt% 1,3 propylene sulfate + 0.5wt% MA (based on the weight of the total formulation).
[0191] Electrolyte preparation: Preparation of 2,2-difluoroethyl acetate (DFEA)
[0192] The 2,2-difluoroethyl acetate used in the following examples was obtained by mixing potassium acetate with HCF 2 CH 2 Prepared by Br reaction. The following is the procedure used for this preparation.
[0193] Potassium acetate (Aldrich, Milwaukee, WI, 99%) was dried at 100° C. under vacuum of 0.5-1 mm of Hg (66.7-133 Pa) for 4 to 5 h. The dried material had a water content of less than 5 ppm as determined by Karl-Fischer...
example 2
[0230] The following example describes the preparation of the following composition: 1M LiPF 6 +4:21:75FEC:PC:DFEA (wt% / wt% / wt%, based on the total weight of FEC, PC and DFEA)+0.85wt% LiBOB+1.5wt% 1,3-propylene sulfate+0.5wt %MA (based on weight of total formulation). By mixing 12.8816 g of 2,2-difluoroethyl acetate, 0.6821 g of fluoroethylene carbonate (FEC, BASF Corporation, Independence, Ohio) and 3.6056 g of propylene carbonate (PC, BASF Inc., Independence, Ohio) assembled the electrolyte for this example in a nitrogen-purged drybox. Molecular sieves (3A) were added and the mixture was dried to less than 1 ppm water and filtered through a 0.25 micron PTFE syringe filter.
[0231] 1.9991 g of the above mixture was combined with 0.0233 g of LiBOB. Stir the material gently to dissolve the components. Add 0.0351 g of 1,3-propylene sulfate, 0.0113 g of MA, and 0.2655 g of LiPF 6 (BASF Corporation, Independence, Ohio). The standing material was stirred gently to dissolve t...
example 4
[0251] This example describes the preparation of a 10% by weight solution of 1,3 propylene sulfate in FEC. A mixture containing 10 wt% 1,3 propylene sulfate and 90 wt% fluoroethylene carbonate was allowed to age at 25°C. After thirteen days, the solution was slightly colored, but significantly less colored than the comparative solution with ethylene sulfate shown in Comparative Example D below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com