A d-a-π-a type n-hybrid porphyrin-dipyrrolene nonlinear optical material and its synthesis method
A technology of nonlinear optics and optical materials, applied in the field of organic fine chemical industry, can solve the problems of inability to use for a long time, easy photobleaching, difficult to solve its difficulties, etc., and achieves high microscopic first-order hyperpolarizability and photophysical and chemical stability. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
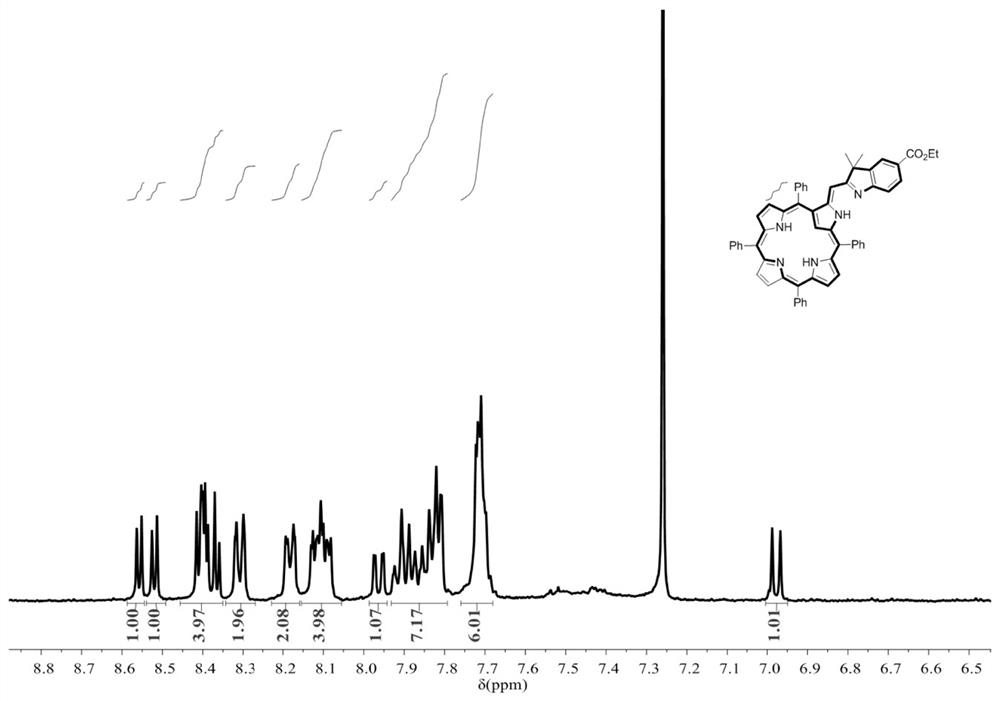
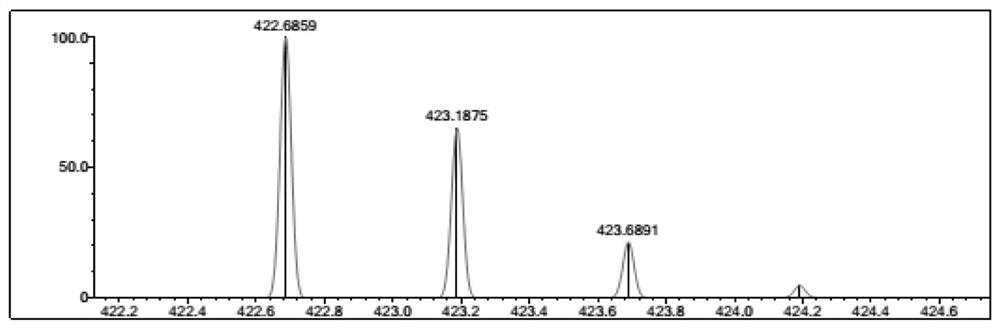
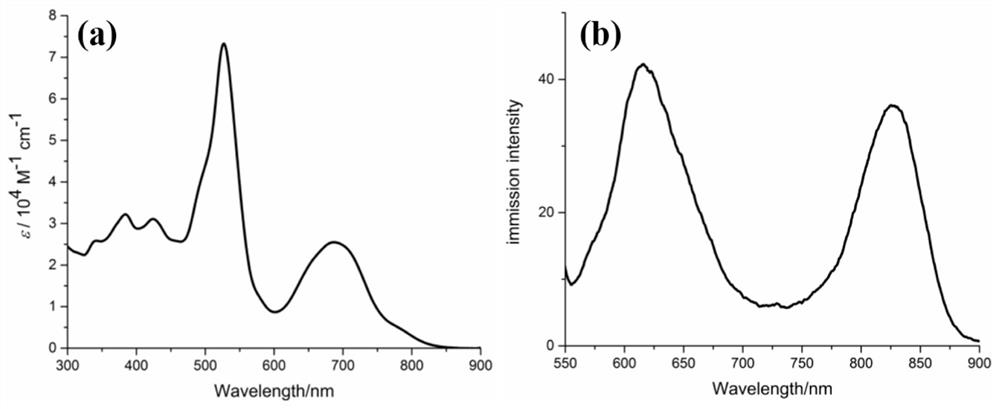
[0027] Take 0.28g (0.12mmol) of 5-ethoxyformyl-2,3,3-trimethylindole and 0.61g (0.10mmol) of 5,10,15,20-tetraphenyl-N-heteroporphyrin In DMF, stirred for 5 minutes, refluxed at 150° C. for 0.5 hours, after the reaction was completed, spin-dried, filtered and purified by column chromatography to obtain a purple compound with a yield of 93%.
[0028]
Embodiment 2
[0030] Take 0.46g (0.2mmol) of 5-ethoxyformyl-2,3,3-trimethylindole and 0.61g (0.10mmol) of 5,10,15,20-tetraphenyl-N-hybrid porphyrin In DMF, stirred for 5 minutes, and refluxed at 150°C for 0.5 hours. After the reaction was completed, the purple compound was obtained by filtration and purification column chromatography with a yield of 92%.
[0031]
Embodiment 3
[0033] Take 0.23g (0.1mmol) of 5-ethoxyformyl-2,3,3-trimethylindole and 0.61g (0.10mmol) of 5,10,15,20-tetraphenyl-N-hybrid porphyrin In DMF, stirred for 5 minutes, and refluxed at 150°C for 0.5 hours. After the reaction was completed, the purple compound was obtained by filtration and purification column chromatography with a yield of 84%.
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com