Inhibitors of the fibroblast growth factor receptor in combination with cyclin-dependent kinase inhibitors
A technology of growth factor receptors and fibroblasts, which is applied in the field of cancer treatment of subjects, and can solve the problems of lack of kinase selectivity and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0253] 1. A method of treating cancer in a patient in need thereof comprising administering to the patient a therapeutically effective amount of at least one fibroblast growth factor receptor 4 (FGFR4) inhibitor and at least one cyclin-dependent kinase ( combination of CDK) inhibitors.
[0254] 2. The method of embodiment 1, wherein said at least one CDK inhibitor is selected from CDK4 / 6 inhibitors.
[0255] 3. The method of embodiment 1 or 2, wherein said at least one CDK inhibitor is selected from the group consisting of palbociclib, 7-cyclopentyl-N,N-dimethyl-2-((5-(piperciclib Azin-1-yl)pyridin-2-yl)amino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide, 2-(2-chlorophenyl)-5,7-dihydroxy- 8-[(3S,4R)-3-Hydroxy-1-methyl-4-piperidinyl]-4-benzopyrone, N-(5-((4-ethylpiperazin-1-yl )methyl)pyridin-2-yl)-5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl)-1H-benzo[d]imidazol-6-yl)pyrimidine-2 -Amines, GZ38-1 and pharmaceutically acceptable salts thereof.
[0256] 4. The method according to a...
Embodiment 1
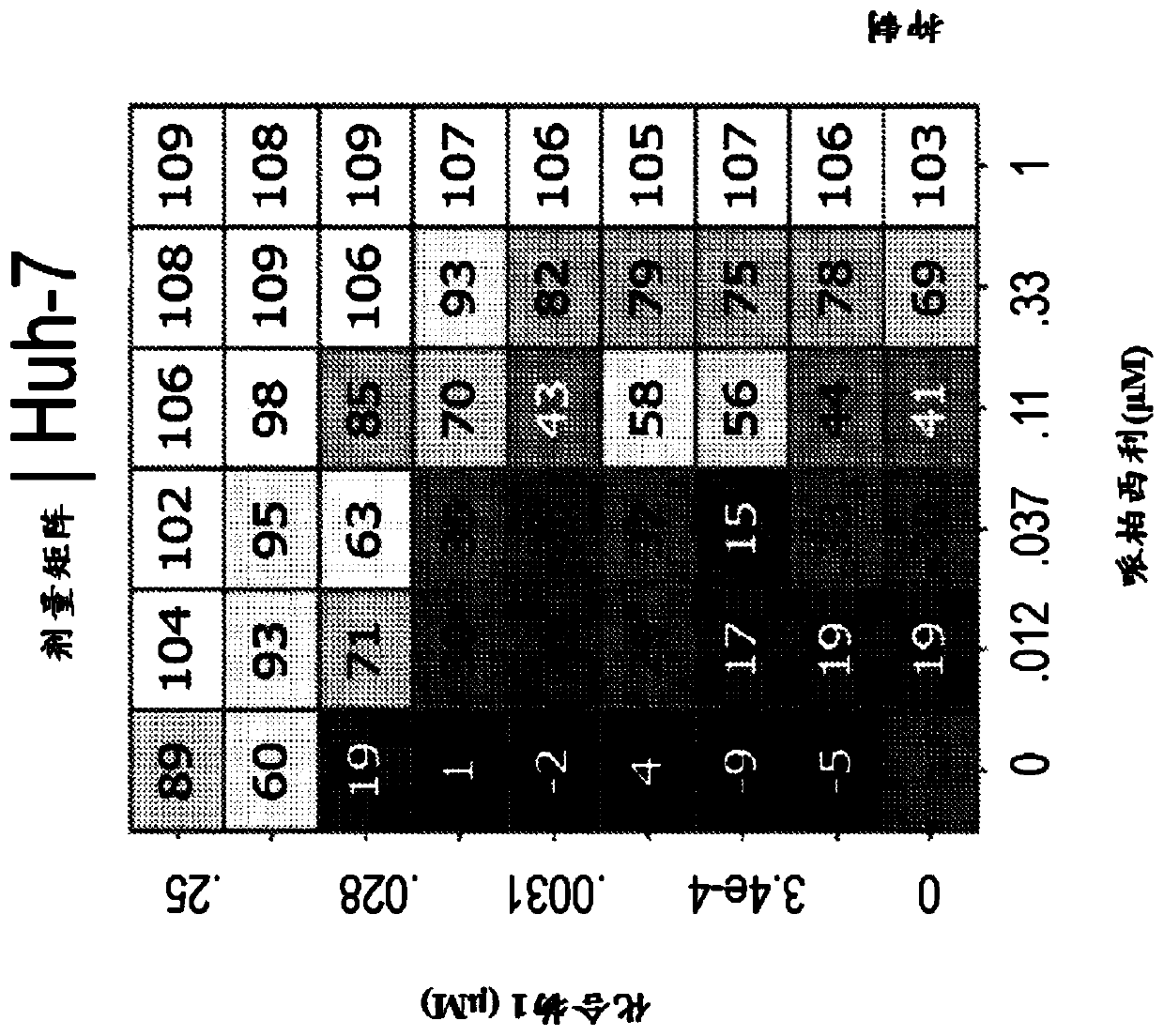
[0309] Example 1: Combination studies of palbociclib and compound 1 in cells
[0310] Combinations of FGFR4 and CDK4 / 6 inhibitors were evaluated in several signal-seeking cell-based in vitro assays (data not shown). Use a variety of different standard antiproliferative assays such as MTS, MTT, and Cell Titer to filter. Many of the cell lines tested showed sensitivity, including in some cases partial responses, such as ZR-75-1, SW1116, TE-8, SNU-761, SNU-878, or in some cases synergistic Reactions such as JHH7, MDA-MB-453, Huh-7. Not all cell lines exhibit sensitivity, which may be due to various reasons. For example, lack of sensitivity is observed in cell lines that are resistant to either agent alone, such as cell lines that do not have an intact FGFR4 signaling pathway (eg, JHH4). In other cell lines, limitations related to the in vitro assay format or readout time points may affect activity. As previously reported, palbociclib activity may be weak in short-term ass...
Embodiment 2
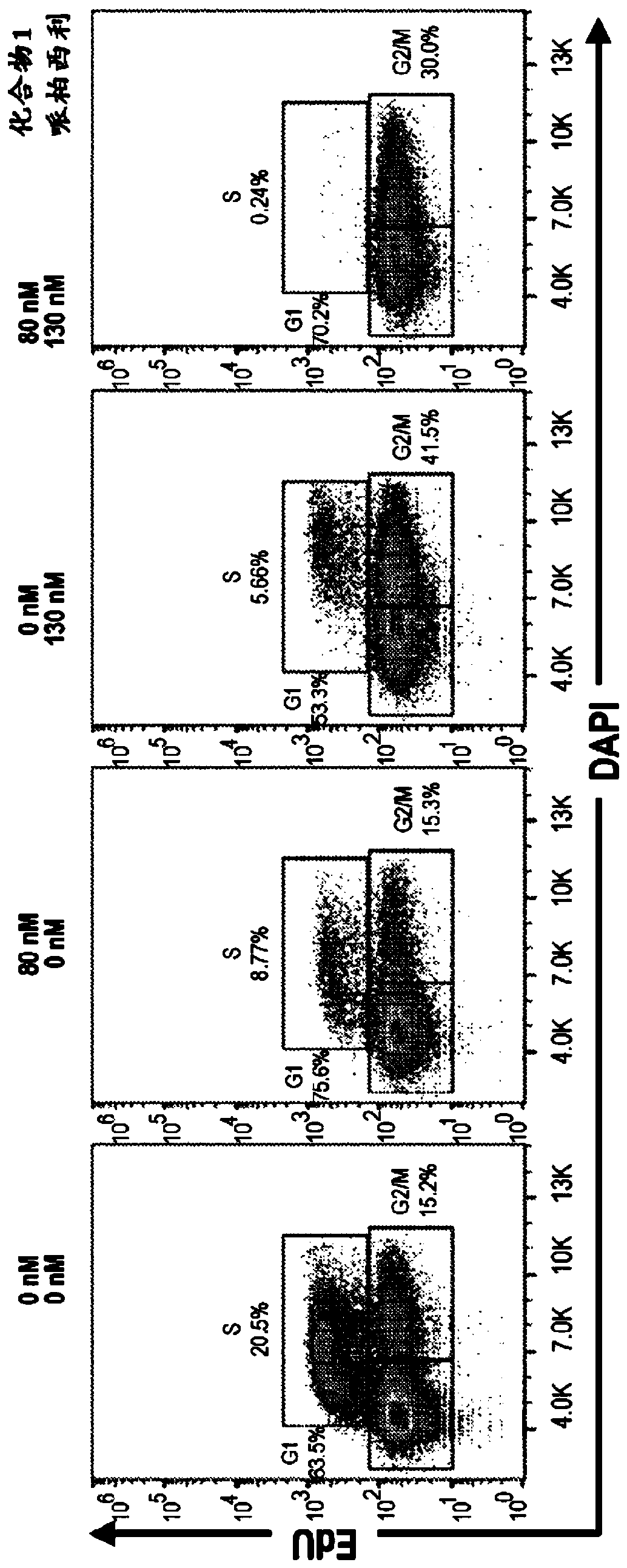
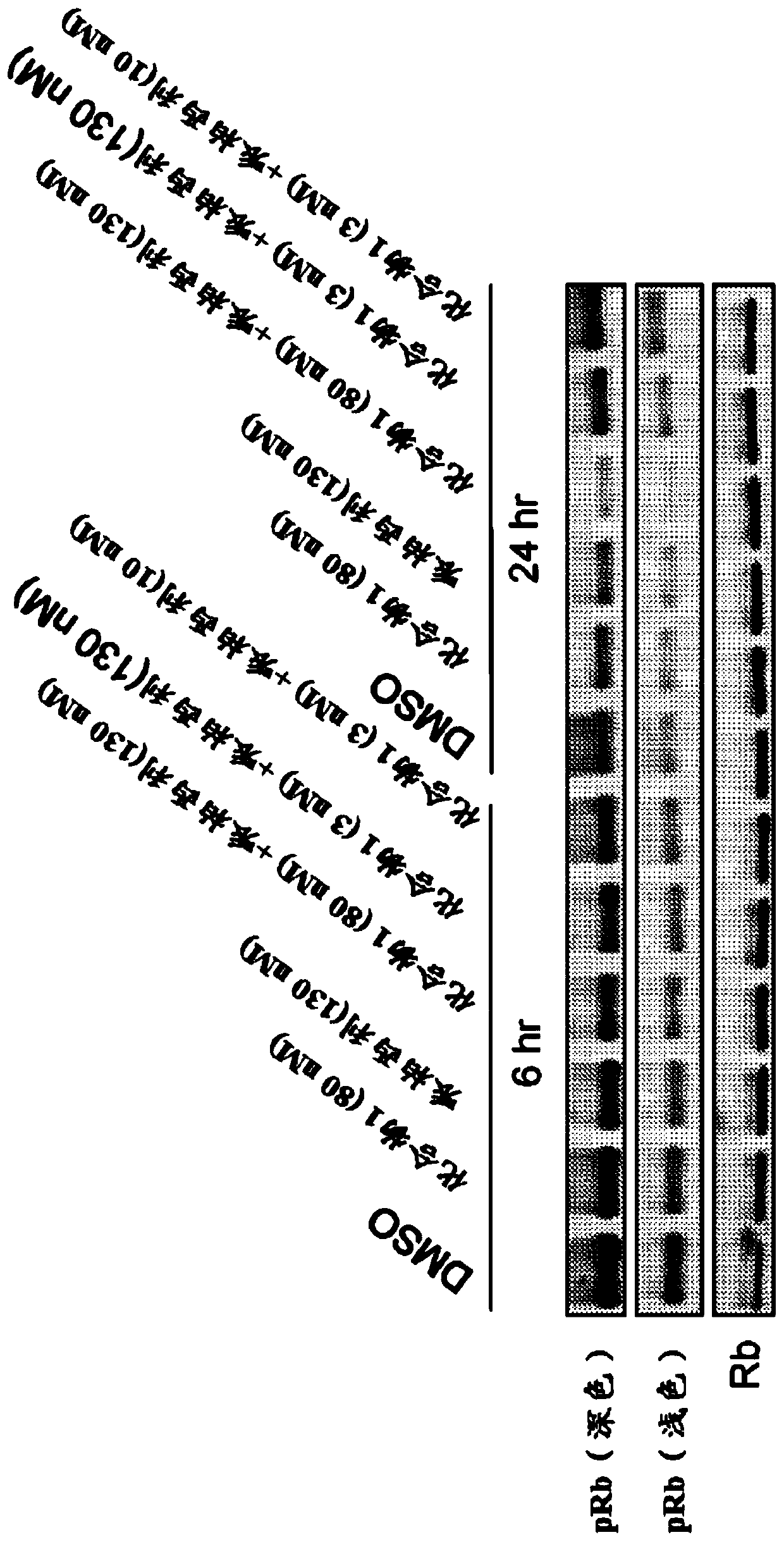
[0315] Example 2: Growth Inhibition of Cells Treated with Palbociclib and / or Compound 1
[0316] Huh-7 cells were treated with the indicated concentrations of Compound 1, Palbociclib, or the combination of Compound 1 and Palbociclib for 72 hours. After compound incubation, Edu was added to the medium at a final concentration of 10 μM for 2 hours. Cells were then harvested and washed with 1% BSA in PBS to pellet the cells and resuspended in 100 μL of Click-iT TM Fixative (Invitrogen, Click-iT TM Plus EdU Flow Cytometry Assay Kit). Cells were incubated with fixative for 15 minutes at room temperature in the dark. Then, wash the cells as previously described and resuspend them in 100 μL 1XClick-iT TM Saponin-based permeabilization-wash reagent and incubate with the reagent at room temperature for 15 minutes in the dark. Then, add 500 μL Click-iT TM Plus reaction mix, incubate cells at room temperature for 30 minutes in the dark. Then, with 3mL 1XClick-iT TM Saponin-Ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com