Antibodies for siglec-15 and methods of use thereof
A technology of antibodies and antigens, applied in the direction of antibodies, antibody medical components, chemical instruments and methods, etc., can solve problems such as functions that have yet to be characterized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
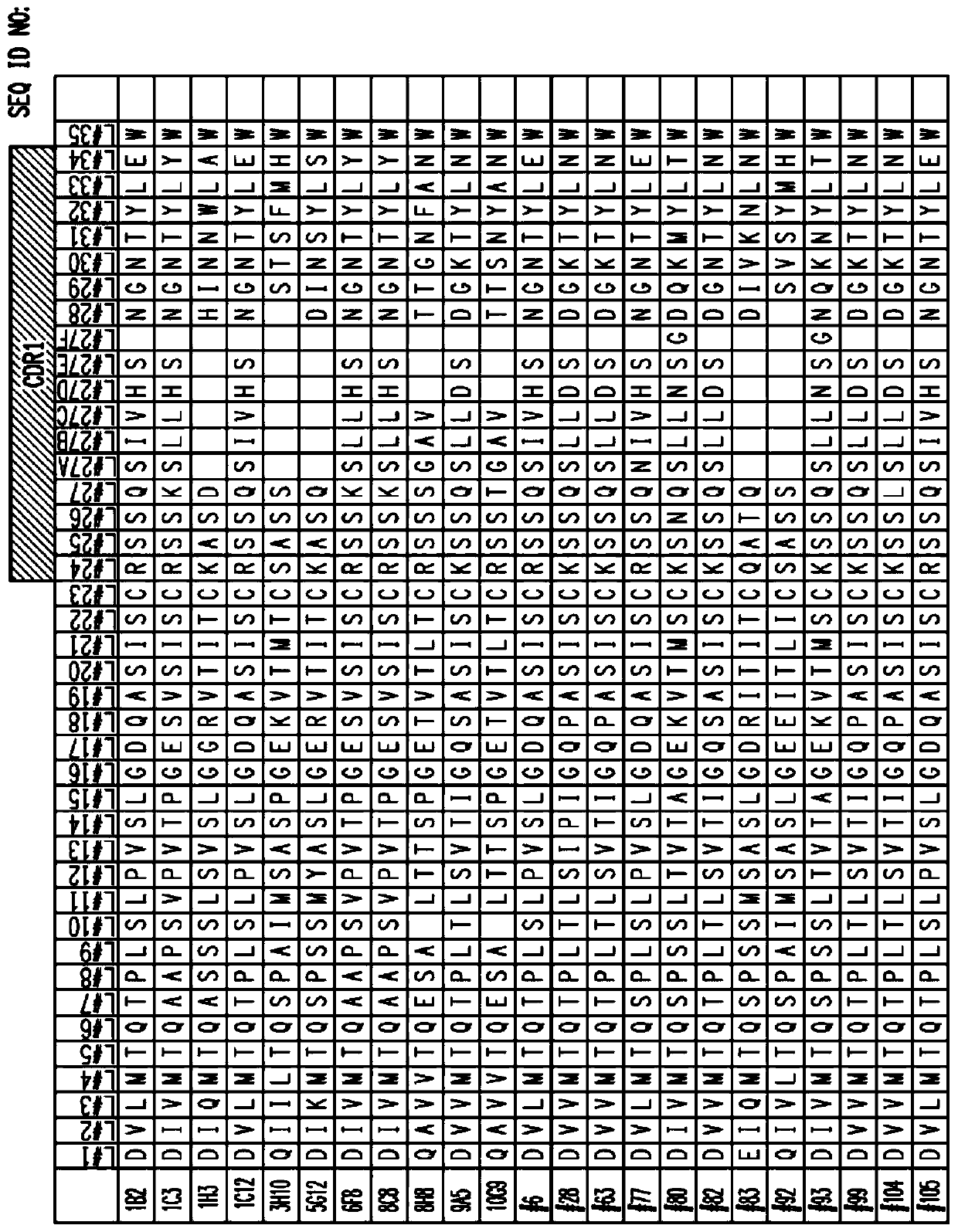
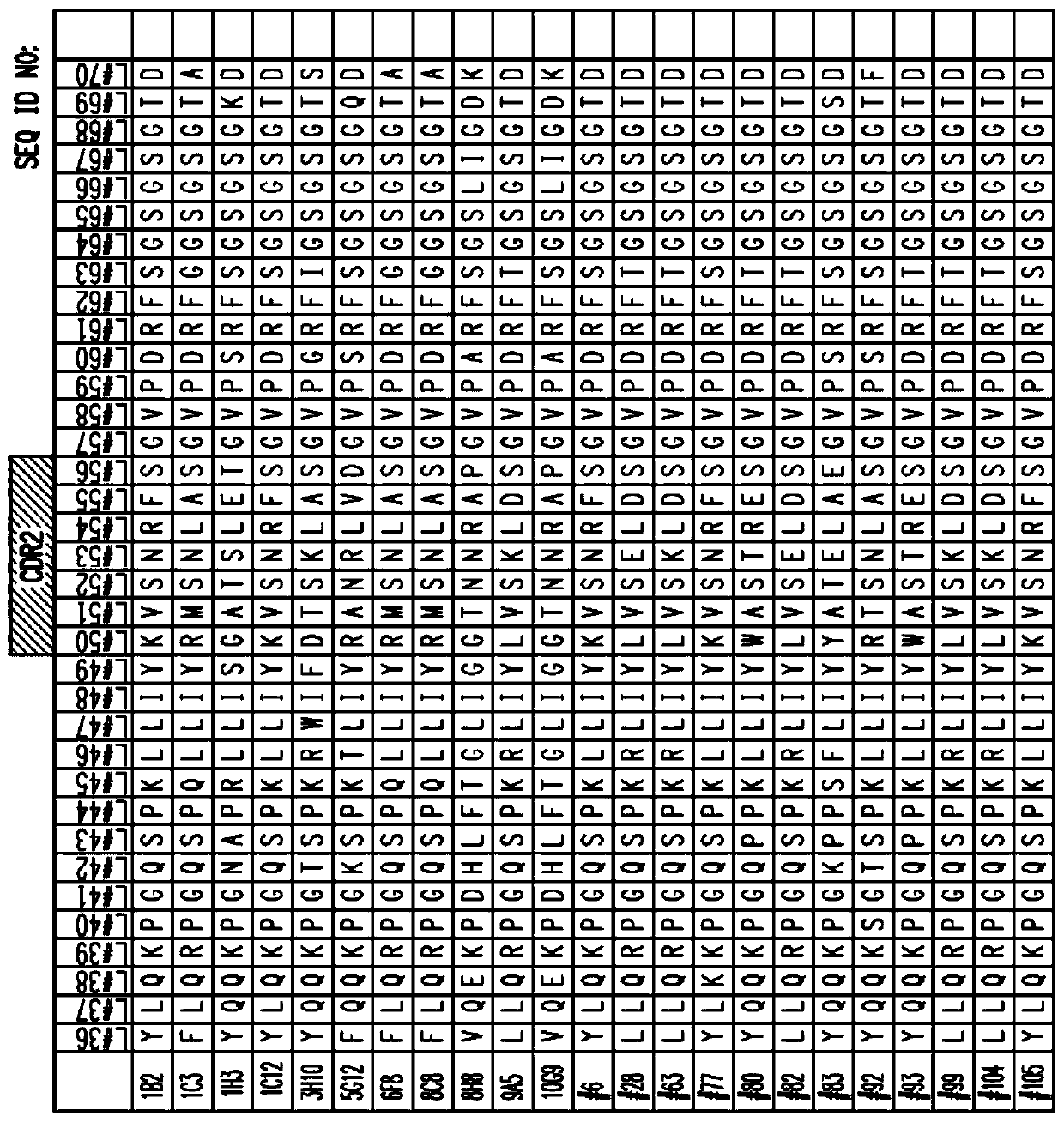
[1001] Example 1: SIGLEC-15 antibody and its heavy and light chain sequences
[1002] Materials and methods
[1003] Mouse anti-human Siglec-15 monoclonal antibody
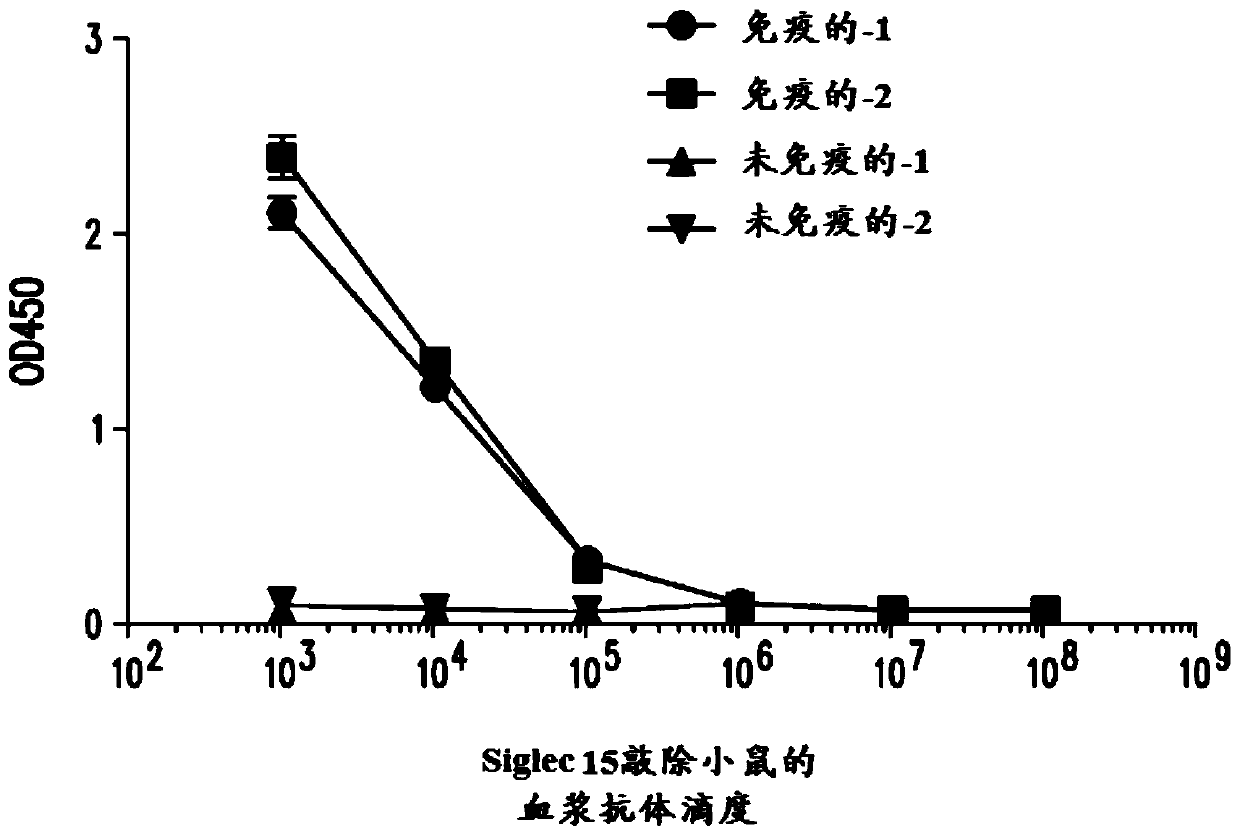
[1004]Siglec-15 knockout mice (n=2) were immunized with hS15.mIg (human Siglec15 extracellular domain [ECD] fused to mouse IgG2a) emulsified with CFA (Complete Freund's Adjuvant). Mice also received injections of GM-CSF and anti-CD40. Mice were challenged 2 weeks later with the same immunogen. Antiserum titers were assessed by assaying sera collected from tail bleeds in hS15.hIg (human Siglec15 ECD fused to human IgG1) coated ELISA plates at various dilutions from 1:1000 to 1:100,000,000 . figure 1 Anti-hS15 antibodies are shown to be detected at >1:100,000 dilutions. The mice received a third dose of antigen two weeks later. Three days after the last boost, mouse splenocytes were harvested and resuspended in RPMI supplemented with 10% FBS and glutamine, and later fused to form hybridomas.
[1005] Electr...
Embodiment 2
[1227] Example 2: Anti-huS15 Antibody Binding to Cells Expressing Human S15 or Mouse S15
[1228] Materials and methods
[1229] Human 293T and mouse CRC MC38 tumor cell lines were transduced with lentiviral vectors carrying human Siglec15 or mouse Siglec15. Cells were sorted to establish human S15 and mouse S15 stable cell lines. 293T.hS15 and MC38.mS15 stable cells were resuspended in FACS buffer and Fc receptors were blocked before incubation with purified Siglec-15 mAb. Aliquot 1E05 cells in 100 μL FACS buffer to separate tubes and add 1 μg purified mAb. Cells were incubated at 4°C for 30 min and then washed twice with excess FACS buffer. Cells were resuspended in 100 μL FACS buffer, and 0.005 μg anti-mouse IgG-PE secondary antibody was added to the samples, incubated for 30 min and washed twice with excess FACS buffer. Cells were fixed in fixation buffer and then analyzed by flow cytometry.
[1230] The assay is Figure 4A shown in .
[1231] result
[1232] Ant...
Embodiment 3
[1234] Example 3: Purified Antibodies Bind Mouse and Human Siglec-15
[1235] Materials and methods
[1236] 293T cells (Operetta) transiently transfected with S15-TM
[1237] K562 cells transiently transfected with S15-TM (FACS)
[1238] 293T cells were transiently transfected with murine Siglec-15 plasmid DNA using the Lipofectamine system, and K562 cells were transfected with human Siglec-15 plasmid DNA by electroporation. 1e5 transfected cells in 100 μl FACS buffer (PBS with 0.5% serum) were aliquoted into separate tubes and 1 μg of purified mAb was added. Cells were incubated at 4°C for 30 min and then washed twice with excess FACS buffer. Cells were resuspended in 100 μL FACS buffer, and 0.005 μg anti-mouse IgG-PE secondary antibody was added to the samples, incubated for 30 min and washed twice with excess FACS buffer. Cells were fixed in fixation buffer (2%) and then analyzed by flow cytometry.
[1239] U87 cells were resuspended in FACS buffer and Fc receptors ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com