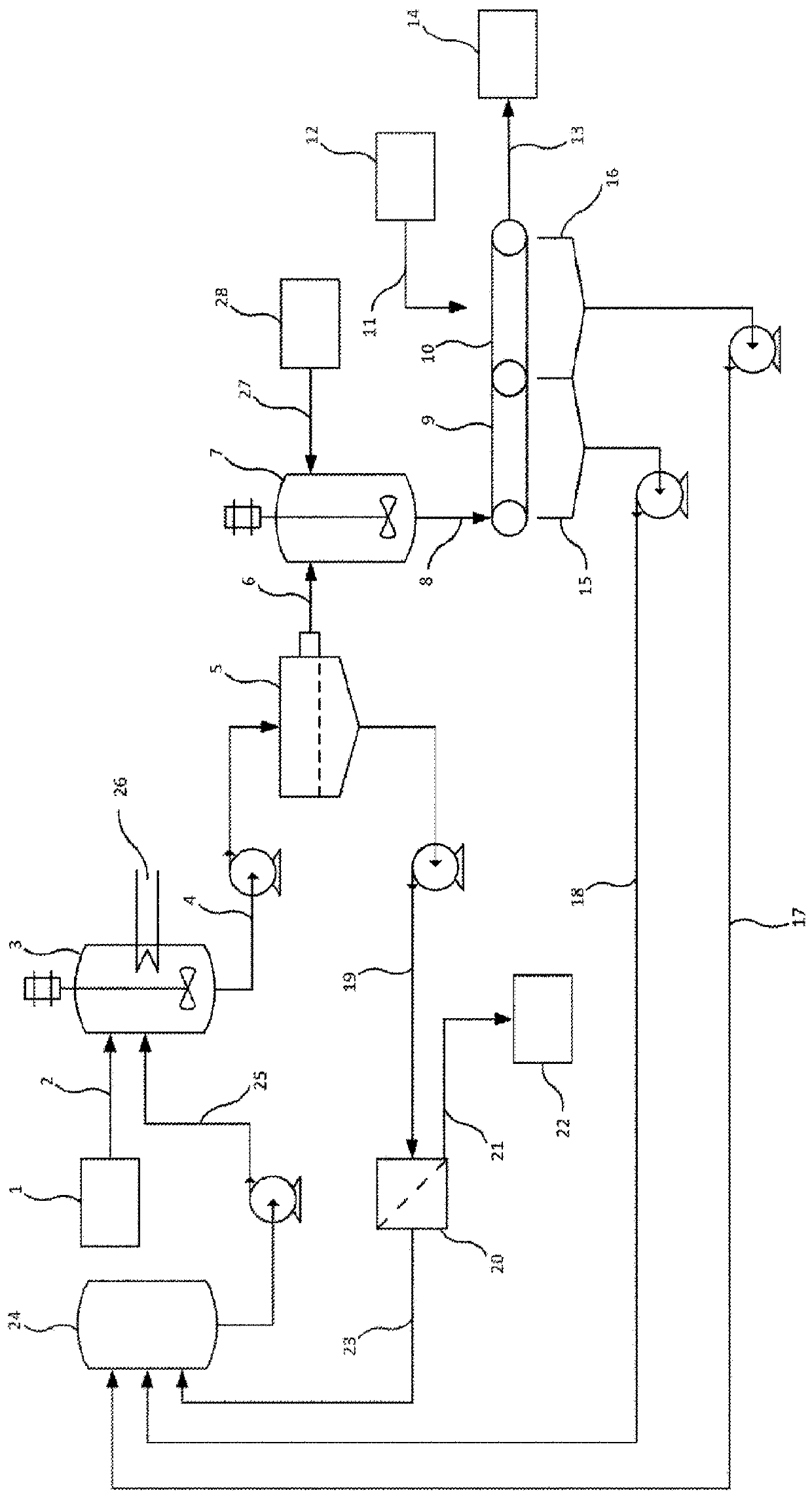
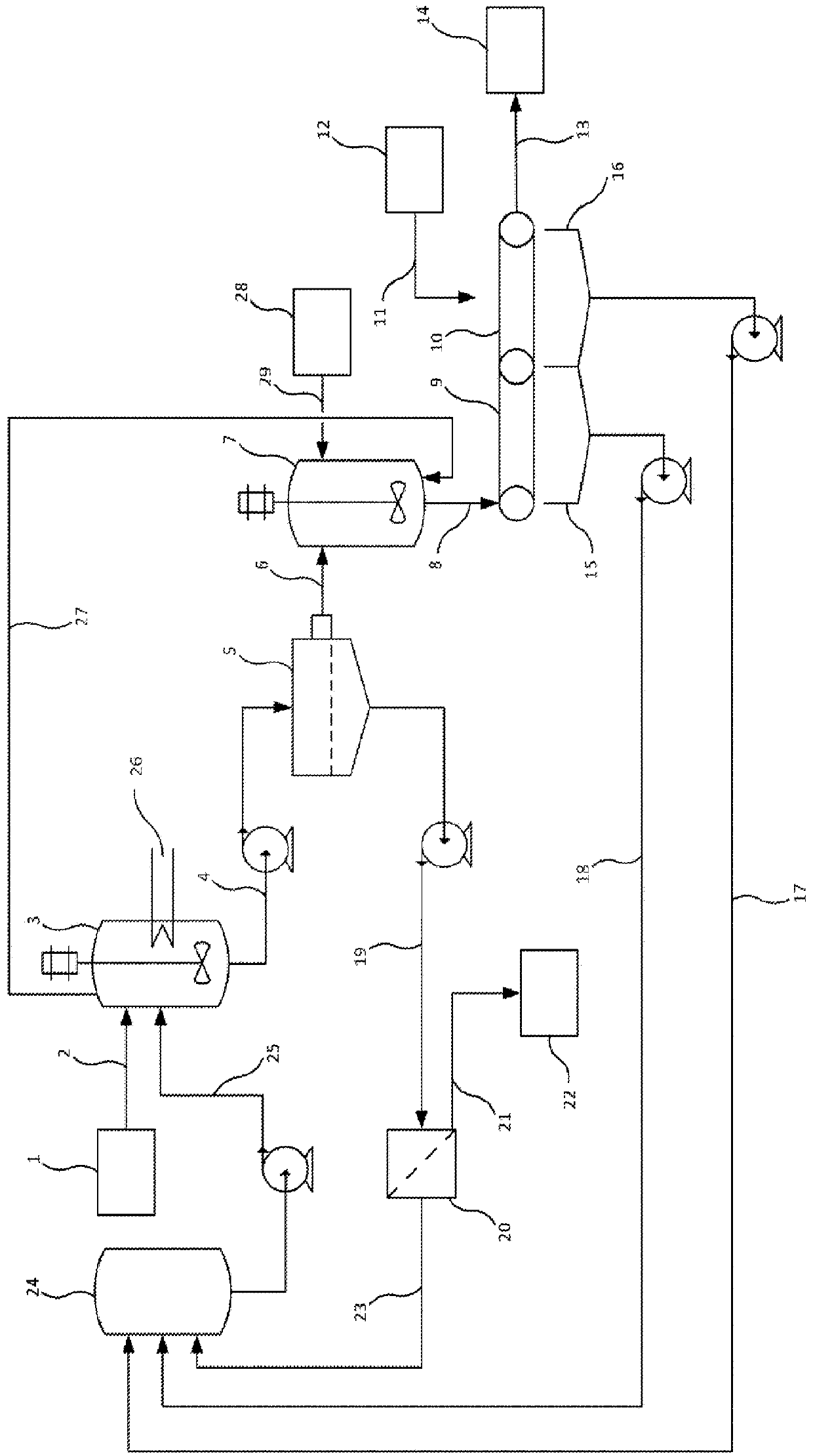
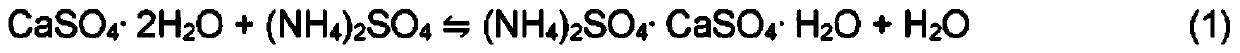Releasing impurities from calcium-based mineral
A mineral, calcium-based technology, used in calcium/strontium/barium compounds, calcium/strontium/barium oxides/hydroxides, ammonia compounds, etc., can solve the problems of expensive materials, increased maintenance costs, and high costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1: Effect of Salt Concentration.
[0118] The gypsum used in this example came from the mine and was ground to a particle size of less than 250 microns and an initial brightness value of 64.2%.
[0119] Tested under the following conditions:
[0120] The salt used in the ionic saline solution is ammonium sulfate.
[0121] Gypsum – 50g
[0122] Temperature – 102°C
[0123] Water Ratio – 1:10
[0124] Dwell time – 30 minutes
[0125]
[0126]
[0127] It is observed that the impurity layer is significantly darker from the concentration of 30% and higher.
[0128] It is believed that the resulting final brightness is optimized when the salt concentration is greater than 25%.
Embodiment 2
[0129] Example 2: Effect of temperature.
[0130] The gypsum used in this example came from the mine and was ground to a particle size of less than 250 microns and an initial brightness value of 64.2.
[0131] Tested under the following conditions:
[0132] Gypsum – 50g
[0133] Salt concentration – 40%
[0134] Water Ratio – 1:10
[0135] Dwell time – 30 minutes
[0136] temperature(℃) Final Brightness (%) 20 64.4 81 76.5 92 88.2 102 93.3
[0137] It is observed that the impurity layer is significantly darker from 92 °C and higher temperatures.
[0138] It is believed that the resulting final brightness is optimized at temperatures of 92°C and higher. However, it has been shown that a temperature of about 85°C will yield a brightness of about at least 80-85%, which may equate to a purity of about 90%, and thus may be sufficient for some uses.
Embodiment 3
[0139] Example 3: Effect of the ratio of gypsum to water.
[0140] The gypsum used in this example came from the mine and was ground to a particle size of less than 250 microns and an initial brightness value of 64.2.
[0141] Tested under the following conditions:
[0142] Gypsum – 50g
[0143] Salt concentration – 40%
[0144] Temperature – 102°C
[0145] Dwell time – 30 minutes
[0146] ratio Final Brightness (%) 1:2 89.2 1:5 90.1 1:10 93.3 1:20 97.4
[0147] It is believed that increasing the ratio of ammonium sulfate solution to gypsum improves the brightness of the resulting gypsum.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com