A kind of synthetic method of heteroatom-directed synthesis of benzoquinoline ester derivatives
A technology of benzoquinoline and synthetic method, which is applied in the direction of organic chemistry, can solve the problems of not being suitable for industrial production, many side reactions, and high cost, and achieve the effects of low cost, few side reactions, and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
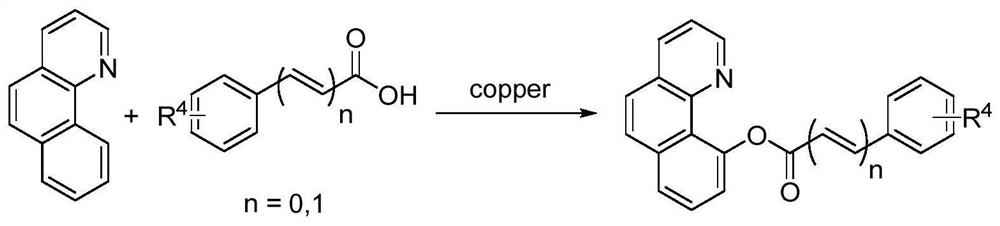
[0029] The invention provides a heteroatom-directed synthesis method for benzoquinoline ester derivatives, which comprises: heating and reacting quinoline derivatives, an organic carboxylic acid, a copper catalyst and a silver salt in an organic solvent under mixed conditions , to obtain benzoquinoline ester derivatives; wherein the quinoline derivatives are benzoquinoline as shown in formula I or 2-phenoxypyridine as shown in formula II; the benzoquinoline esters The structural formula of the derivative is shown in formula III or formula IV, X is the ester group in the organic carboxylic acid;
[0030]
[0031] Through the above technical scheme, the present invention overcomes many defects in the synthesis of quinoline ester derivatives in the prior art, and provides a synthetic method for synthesizing benzoquinoline ester derivatives, using silver salt as an oxidant, cheap Copper catalysts such as (one or more of copper acetate, copper sulfate, cuprous bromide, cuprous o...
Embodiment 1
[0046] The synthesis of 10-benzoquinoline benzoate comprises the following steps:
[0047] A. Take 0.2mmol benzoquinoline and 0.3mmol benzoic acid in the reaction tube, then add 0.04mmol Cu in turn 2 O, 0.4 mmol Ag 2 CO 3 , 3mL PhCl, stirred and reacted at 140°C for 18h.
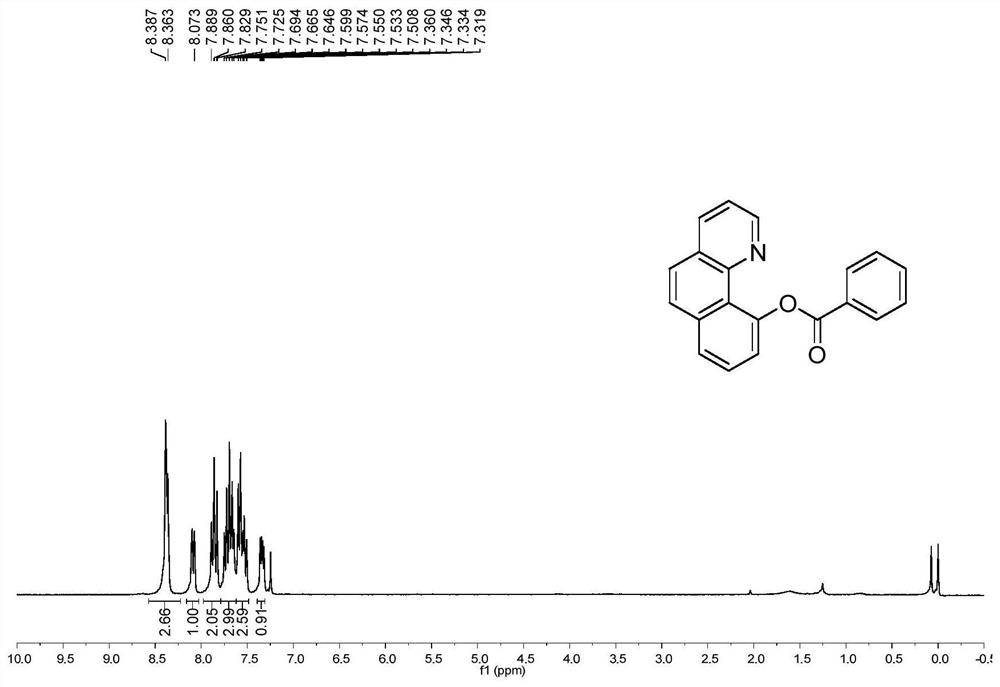
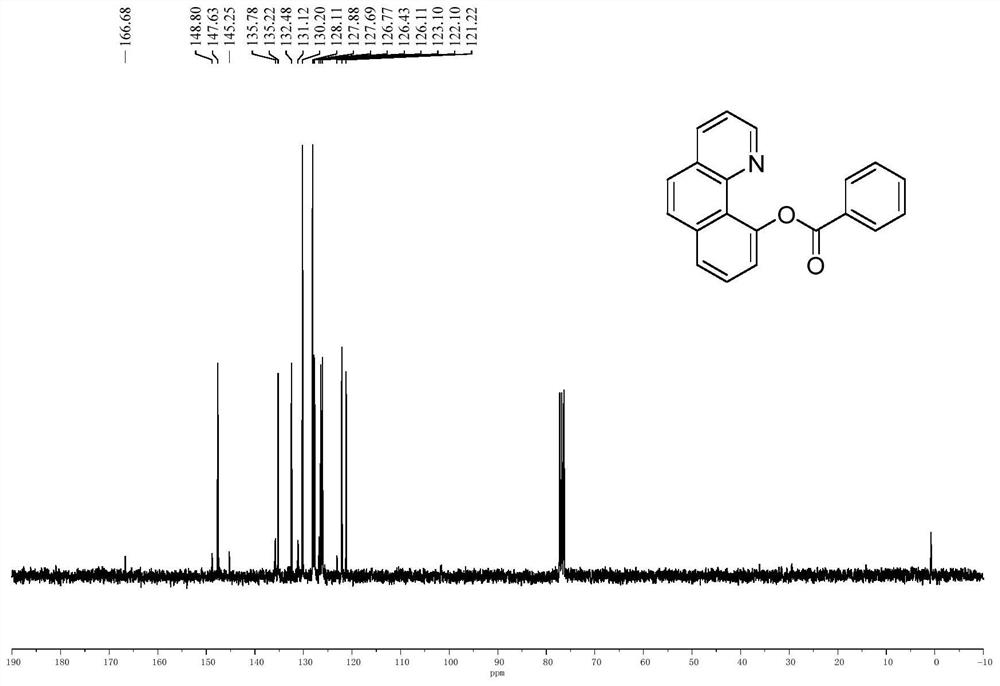
[0048] B. Extract the product with ethyl acetate, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain a crude product, which is purified by silica gel column chromatography (volume ratio of solvent: petroleum ether: ethyl acetate = 10:1) A white solid was obtained. The result is as Figure 2a , Figure 2b As shown, it was confirmed to be 10-benzoquinoline benzoate. Yield 91%.
[0049] 1 H NMR (300MHz, CDCl 3 ): δ8.38(d, J=7.2Hz, 3H), 8.09(d, J=7.8Hz, 1H), 7.86(t, J=9.0Hz, 2H), 7.75-7.65(m, 3H), 7.60 -7.51(m,3H),7.36-7.32(m,1H). 13 C NMR (75MHz, CDCl 3 ): δ166.7, 148.8, 147.6, 145.3, 135.8, 135.2, 132.5, 131.1, 130.2, 128.1, 127.9, 127.7, 126.8, 126.4, 126.1, 123.1,...
Embodiment 2
[0052] The synthesis of 10-benzoquinoline-4-chlorobenzoate comprises the following steps:
[0053] A. Take 0.2mmol benzoquinoline and 0.3mmol 4-chlorobenzoic acid in the reaction tube, then add 0.04mmol Cu in turn 2 O, 0.4 mmol Ag 2 CO 3 , 3mL PhCl, stirred and reacted at 140°C for 18h.
[0054] B. Extract the product with ethyl acetate, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain a crude product, which is purified by silica gel column chromatography (volume ratio of solvent: petroleum ether: ethyl acetate = 10:1) A yellow solid was obtained. The result is as Figure 3a , Figure 3b As shown, it was confirmed to be 10-benzoquinoline-4-chlorobenzoate.
[0055] Yield 87%
[0056] 1 H NMR (500MHz, CDCl3): δ8.38 (dd, J = 4.5, 2.0Hz, 1H), 8.31 (d, J = 8.5Hz, 2H), 8.11 (dd, J = 8.0, 2.0Hz, 1H), 7.90-7.84(m,2H),7.75-7.69(m,2H),7.56-7.50(m,3H),7.36(q,J=4.5Hz,1H). 13 C NMR (125MHz, CDCl3): δ166.7, 149.5, 148.5, 146.1, 139.8, 136.7, 136....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com