Application of furanone compounds in preparation of drugs for preventing or treating hyperlipidemia
A technology for hyperlipidemia and furanone, applied in the field of biomedicine, can solve the problem that furanone compounds do not show cytotoxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] 1. Lipoprotein preparation
[0046] experimental method:
[0047]Lipoproteins were prepared by ultracentrifugation, the plasma density was adjusted to 1.006 g / mL, and ultracentrifuged at 400,000 × g for 24 hours at 10°C. The upper layer containing very low-density lipoprotein was removed, the density of the remaining plasma was adjusted to 1.063 g / mL, and low-density lipoprotein (LDL) was obtained by ultracentrifugation at 400,000 × g for 24 h at 10°C. Draw the upper layer containing low-density lipoprotein (LDL) and place it in the EP tube. The remaining liquid was then readjusted to a density of 1.21g / mL, and ultracentrifuged at 400,000×g for 48 h to obtain high-density lipoprotein (HDL).
[0048] HDL dialysis method: add chelated metal ions containing EDTA-2Na (0.1%, w / v) to PBS solution (pH7.2), so as to reduce oxidation during ultracentrifugation, and dialyze for 24 hours. Dialyze in EDTA-free PBS solution for 24 hours, during which the dialysate was changed 3-5...
Embodiment 1
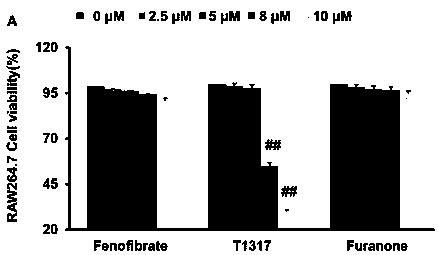
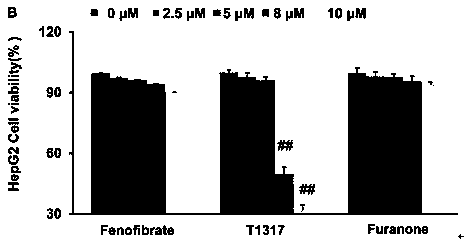
[0050] Example 1 Cytotoxicity assay
[0051] experimental method
[0052] (1) RAW 264.7 macrophages and HepG2 cells in the logarithmic growth phase were first mixed with 1×10 3 cells / mL were inoculated in 96-well plates, and 0.2 mL of 10% DMEM medium was added to each well, and cultured for 12 hours.
[0053] (2) RAW 264.7 macrophages and HepG2 cells were treated with furanone compounds, fenofibrate and LXR activator (T0901317), respectively, and the final concentrations of the drugs were set to 0 μM, 2.5 μM, 5 μM, 8 μM and 10 μM. μM. The medium is DMEM (10% fetal bovine serum) medium. Set more than 3 parallel wells in each group, set at 37°C, 5% CO 2 Continue culturing for 24 h in the incubator.
[0054] (3) After the culture, the supernatant was discarded, and 20 μL of 5.0 mg / mL MTT was added to each well. The cells were incubated for an additional 4 hours to form formazan crystals.
[0055] (4) Carefully discard the supernatant, add 150 μl of DMSO to each well to dis...
Embodiment 2
[0058] Example 2 Oil Red O staining
[0059] experimental method
[0060] (1) RAW 264.7 macrophages or HepG2 cells were mixed with 2×10 cells per well 4 The final density of cells was seeded into 6-well plates. After 12 hours, the medium was aspirated off and the cells were washed 3 times with PBS.
[0061] (2) Then, add 1.5 mL of ox-LDL containing 1% FBS and a final concentration of 50 mg / mL to each well of RAW 264.7 macrophages, and continue to incubate for 24 hours; add 1.5 mL of 1% FBS to each well of HepG2 cells and oleic acid (0.5mM) medium, and continue to incubate for 24 hours.
[0062] (3) After the incubation, gently wash the cells 3 times with PBS. Set blank group (DMEM medium), control group T0901317 (5 μM), and experimental group furanone compounds (5 μM) to treat RAW 264.7 macrophages. Add 500 μl of DMEM medium (containing 1% FBS) containing the above drugs to each well to treat the cells for 4 hours. Groups were set up according to the same experimental me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com