Gene therapy for mucopolysaccharidosis, type ii
A polynucleotide, HIV-1 technology, applied in the field of gene therapy composition, Hunter's syndrome, and treatment of type II mucopolysaccharidosis, can solve the problem of uncured Hunter's syndrome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0269] Construction of the I2S vector
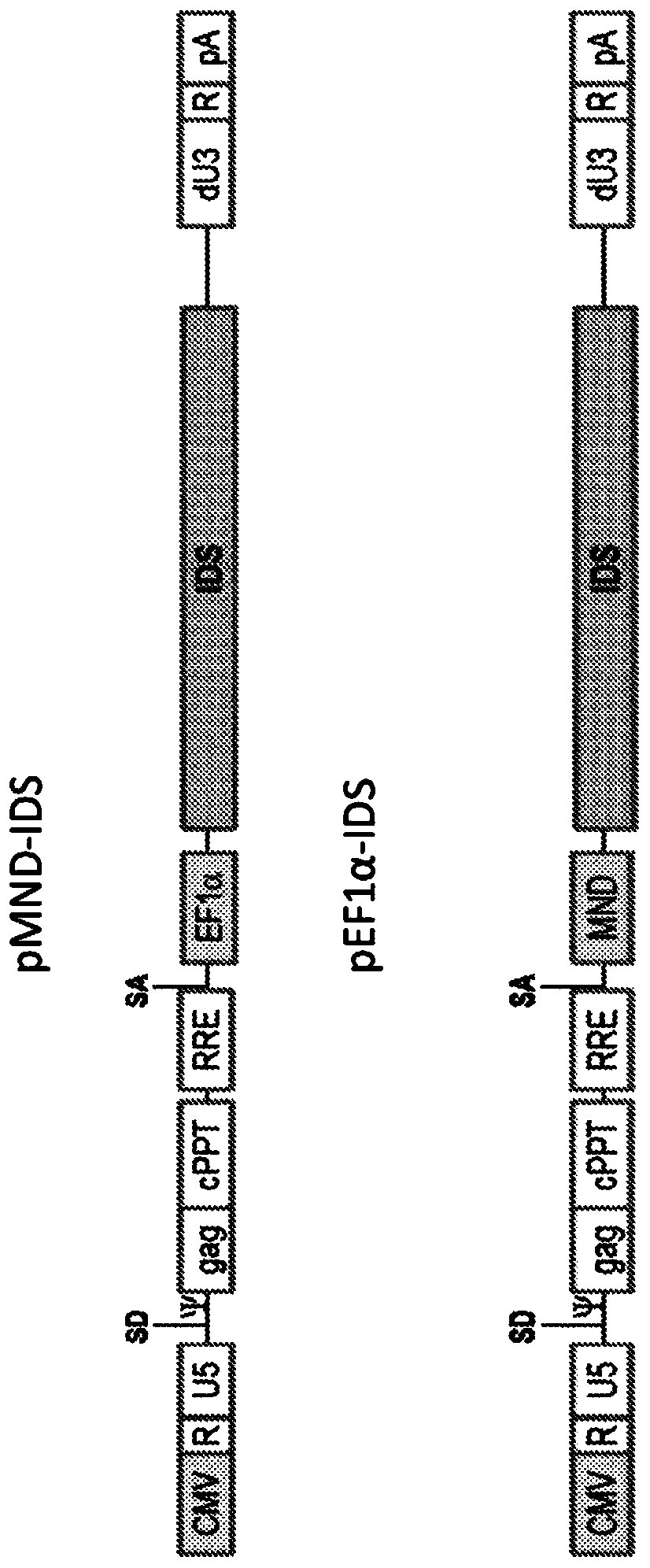
[0270] The following were constructed: a third-generation lentiviral vector containing a chimeric 5′LTR; myeloproliferative sarcoma virus enhancer negative control region deleted (MND) promoter or short elongation factor 1α (EF1α) promoter with dl587rev primer binding site replacement a polynucleotide encoding an iduronate-2-sulfatase (I2S) polypeptide; and a self-inactivating (SIN) 3'LTR. See for example, figure 1 and SEQ ID NO: 1 and 2. Tables 1 and 2 show the identity, Genbank Reference, source name, and citation of each nucleotide fragment of an exemplary lentiviral vector encoding I2S.
[0271] Table 1: pMND-I2S LVV
[0272]
[0273]
[0274]
[0275] Table 2: pEF1α-I2S LVV
[0276]
[0277]
example 2
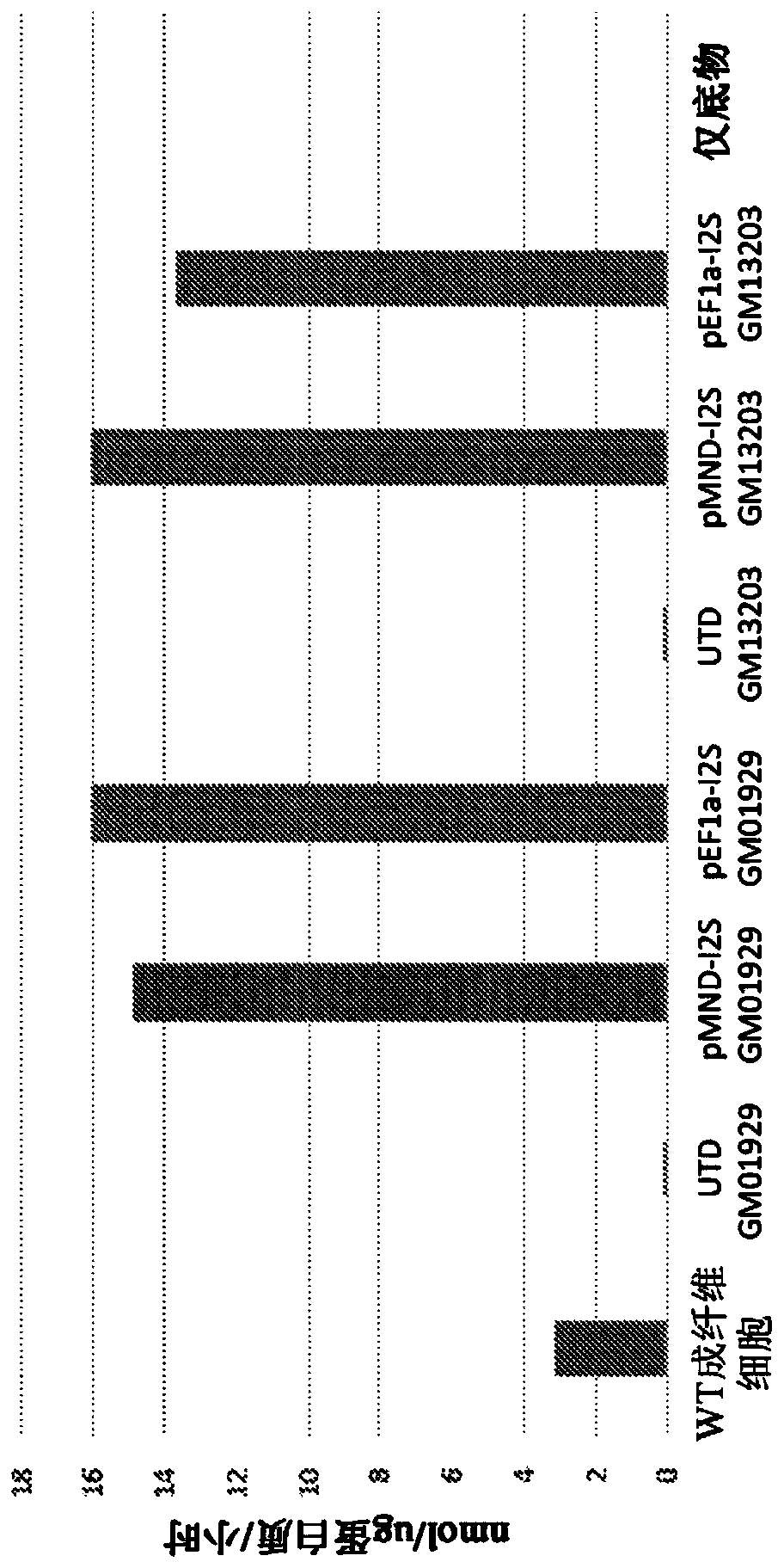
[0279] Fibroblasts transduced with a lentiviral vector encoding I2S
[0280] Human fibroblasts lacking I2S activity due to a homozygous mutation of the I2S gene (I2S - / - Cells) were cultured in Dulbecco's Modified Eagle Medium (DMEM) plus 10% fetal bovine serum (FBS) for twenty-four hours prior to transduction. The cultured I2S- / - Cells were resuspended in DMEM at 5.0E4 cells / ml plus 10% FBS, and 2 mL of this cell suspension was plated into 6-well tissue culture plates and placed at 37°C. Twenty-four hours after cell seeding, cells were transduced with 1 mL of any unpurified lentiviral vector. 1 mL of DMEM plus 10% FBS was added to control wells, and the cells were placed in a 37°C incubator. Twenty-four hours after transduction, a complete media exchange was performed. Forty-eight hours after transduction, 250 uL of supernatant from each well was removed into sterile Eppendorf tubes and frozen at -80°C. Cells were washed with 1 mL of phosphate-buffered saline and lifted w...
example 3
[0282] Protein expression in cells transduced with lentiviral vector encoding I2S
[0283] From wild-type control cells, I2S - / - Cells and I2S transduced with a lentiviral vector encoding I2S - / - Frozen cell pellets of cells (pMND-I2S and pEF1α-I2S) were thawed on ice for Western blotting. To each cell pellet was added 300 μL Mammalian Protein Extraction Reagent and 3 μL 100X HALT Protease Inhibitor Cocktail (Thermo Fisher Scientific). The pellet was resuspended by pipetting up and down slowly, and the cells were incubated on a plate shaker at room temperature for 10 minutes. Cells were centrifuged at 14,000 rpm for fifteen minutes at 4°C, and the supernatant was removed into sterile Eppendorf tubes. Loading dye was prepared by adding 25 μL of β-mercaptoethanol to 475 μL of 4X Laemmli sample buffer (Bio-Rad). Mix the samples at a 3:1 sample:loading dye ratio, 30 µL of prepared loading dye:90 µL of sample. 20 μL of each sample and 8 μL of a Precision Plus Protein Kaleidosc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com