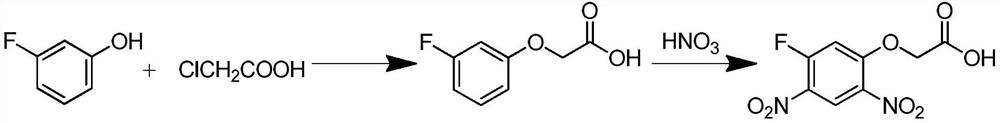
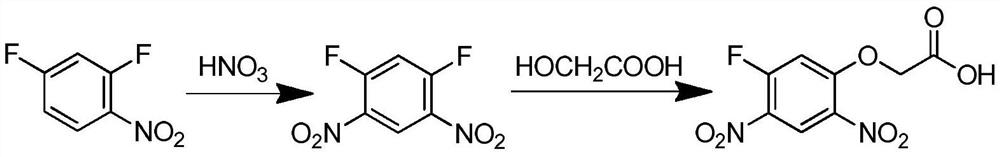
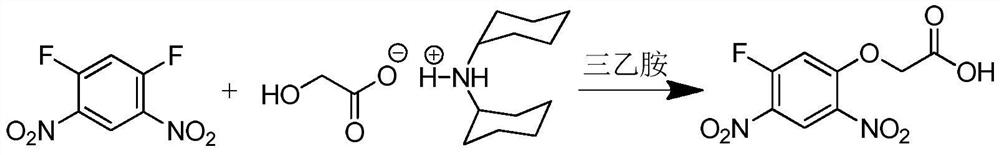
A kind of preparation method of 2-(5-fluoro-2,4-dinitrophenoxy)acetic acid
A technology of dinitrophenoxy and dinitrobenzene, which is applied in the field of preparation of 2-acetic acid, can solve the problems of high production cost, high price, unfavorable commercial production, etc., and achieve short reaction time, less waste, and post-treatment simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 6.12g of 1,5-difluoro-2,4-dinitrobenzene, 15mL of ethylene glycol, and 3.03g of triethylamine into the reactor, and react at room temperature for 2.5h. After the reaction, add 20ml of water, extract with 50ml of toluene, wash the organic layer once with 20ml of water, collect the organic phase and obtain 2-(5-fluoro-2,4-dinitrophenoxy)ethanol with a yield of 84% ; 1 H NMR (500MHz, CDCl 3 )δ8.81(d, J=7.8Hz, 1H), 7.04(d, J=11.9Hz, 1H), 4.44–4.29(m, 2H), 4.08(dt, J=5.8, 4.6Hz, 2H), 2.40 (t, J=6.3Hz, 1H).
Embodiment 2
[0029] Add 10.2g of 1,5-difluoro-2,4-dinitrobenzene, 14mL of ethylene glycol, and 4.24g of anhydrous sodium carbonate into the reactor, and react at room temperature for 3h. After the reaction, add 30ml of water, extract with 55ml of toluene, wash the organic layer once with 20ml of water, collect the organic phase and obtain 2-(5-fluoro-2,4-dinitrophenoxy)ethanol with a yield of 86% .
Embodiment 3
[0031] Add 12.2g of 1,5-difluoro-2,4-dinitrobenzene, 23mL of ethylene glycol, and 5.05g of triethylamine into the reactor, and react at room temperature for 2h. After the reaction, add 35ml of water, extract with 55ml of toluene, wash the organic layer once with 30ml of water, collect the organic phase and obtain 2-(5-fluoro-2,4-dinitrophenoxy)ethanol with a yield of 85% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com