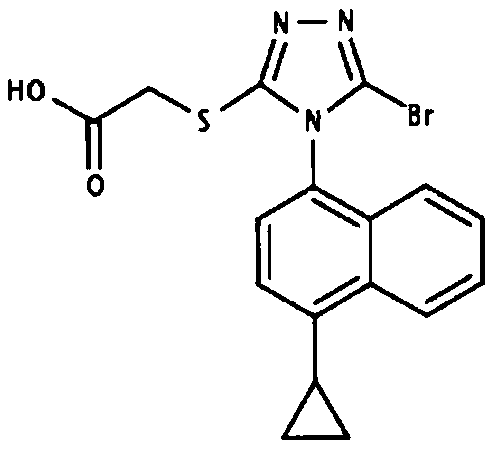
Application of febuxostat in preparation of drugs to treat or prevent Cushing's syndrome
A technology for Cushing's syndrome and febuxostat, which is applied in the application field of febuxostat in the adjuvant treatment of Cushing's syndrome, can solve problems such as undisclosed febuxostat, and achieves reduction of various indicators, Improve the quality of life and improve the effect of disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The therapeutic effect of embodiment 1 febuxostat on Cushing's syndrome
[0020] 1. Materials and methods
[0021] 1.1 Experimental objects and grouping
[0022] 20 patients with Cushing's syndrome were randomly hospitalized (Cushing group), including 6 males and 14 females, with an average age of (30.2±8.2) years and a medical history of 9 months to 15 years. In the past 6 months, he has not taken drugs that affect blood sugar, blood lipids, and blood uric acid metabolism. There was no statistically significant difference between the two groups in terms of ethnicity, gender, age, blood pressure level, body mass index (BMI) and other general information (P>0.05), and they were comparable.
[0023] 1.2 Treatment methods
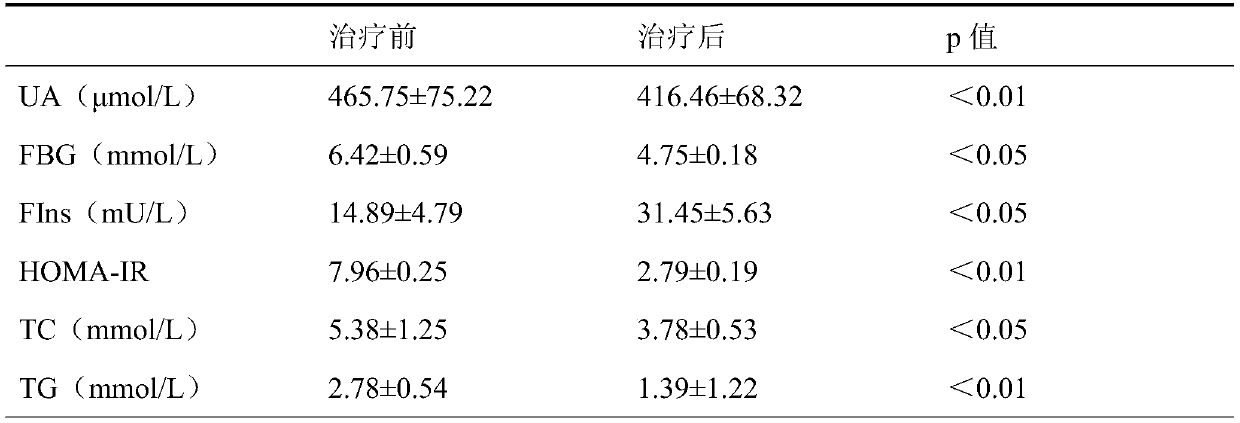
[0024] For the Cushing group, febuxostat was administered orally, once a day, 80 mg each time. Fasting venous blood was collected for 12 h in the morning before administration and 2 months after administration, and blood UA, fasting blood glucose (F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com