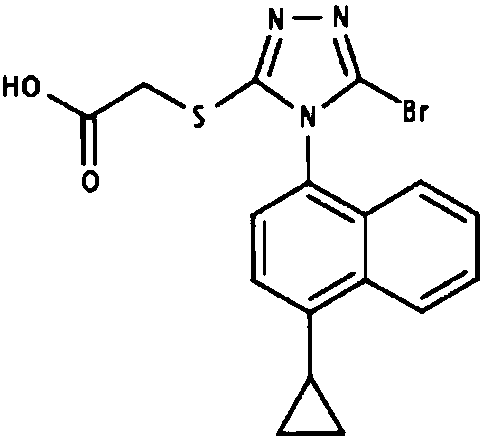
Applications of lesinurad or pharmaceutically acceptable salt thereof in preparation of drugs for treatment or prevention of Cushing's syndrome
A technology for Cushing's syndrome and medicine is applied in the application field of lesinurad or a pharmaceutically acceptable salt thereof in the preparation of medicines for treating or preventing Cushing's syndrome, so as to improve the quality of life, reduce various indicators, and improve the disease condition. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The therapeutic effect of lesinurad on Cushing's syndrome
[0027] 1. Materials and methods
[0028] 1.1 Experimental objects and grouping
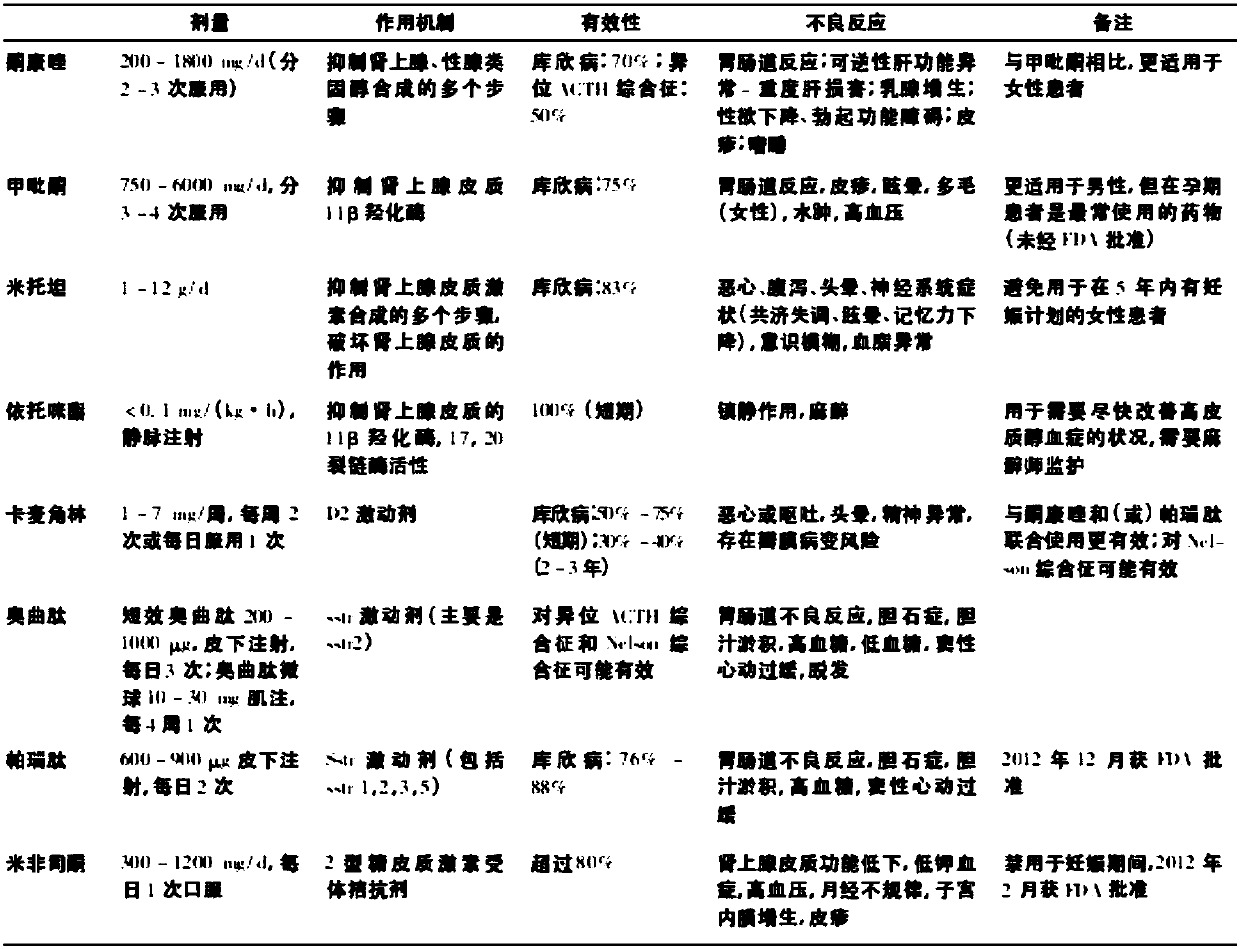
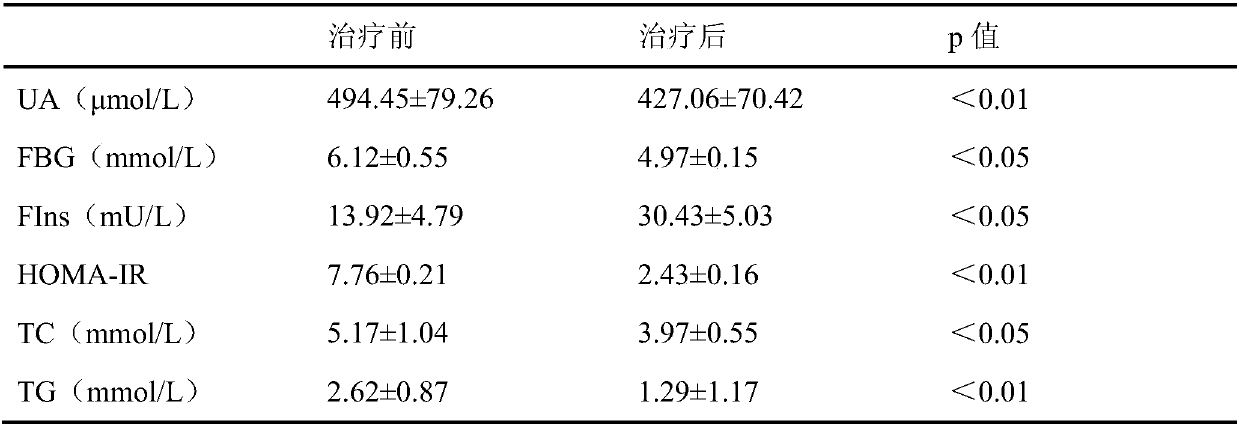
[0029] 22 patients with Cushing's syndrome were randomly hospitalized (Cushing group), including 5 males and 17 females, with an average age of (40.25±10.24) years and a medical history of 6 months to 10 years. Among them, there were 14 cases of Cushing's disease, 6 cases of adrenal sebaceous adenoma, 1 case of ACTH-independent macronodular hyperplasia, and 1 case of ectopic ACTH syndrome. The diagnosis was made based on the patient's clinical manifestations, cortisol, blood ACTH, dexamethasone suppression test, adrenal CT, pituitary MRI and other examinations, and some patients were confirmed by pathological examination after surgery. In the past 3 months, he has not taken drugs that affect blood sugar, blood lipids, and blood uric acid metabolism. There was no statistically significant difference between the two groups in term...
Embodiment 2
[0040] Therapeutic effect of lesinurad combined with drugs that specifically act on pituitary adenomas to inhibit ACTH secretion and rosiglitazone on Cushing's syndrome
[0041] In vitro experiments have confirmed that high concentrations of PPARγ receptor agonists such as rosiglitazone [150mg / (kg·d)] can inhibit the proliferation of mouse AtT20 tumor cells cultured in vitro, and 69% of the tumor-planted mice did not Cushing's syndrome appeared, which also reduced ACTH levels by 65% and cortisol levels by 91%.
[0042] In vitro experiments have confirmed that high concentrations of PPARγ receptor agonists such as rosiglitazone [150mg / (kg·d)] combined with lesinurad [800mg / (kg·d)] can inhibit the proliferation of mouse AtT20 tumor cells cultured in vitro, In mice implanted with tumors, 81 percent were free of Cushing's syndrome, while ACTH levels were reduced by 77 percent and cortisol levels were reduced by 97 percent.
Embodiment 3
[0044] Combination of lesinurad with cortisol synthesis-inhibiting drugs acting on adrenal tumors and trolosteine in the treatment of Cushing's syndrome
[0045] Trilosteine is a competitive inhibitor of the cholesterol synthase 3-beta hydroxysteroid dehydrogenase, which blocks the conversion of pregnenolone to progesterone, ultimately reducing the synthesis of cortisol, aldosterone, and androstenedione. In 7 patients with Cushing's disease, the application of trilosteine can reduce the level of steroids, serum cortisol and 17-hydroxysteroids can be reduced by 50%, and urinary free cortisol can be reduced by 70%. Another 7 patients with Cushing's disease were treated with the combination of trilosteine and lesinurad, and found that serum cortisol and 17 hydroxysteroids could be reduced by 59%, and urinary free cortisol could be reduced by 79%.
[0046] 3 Conclusion
[0047] The pathological basis of metabolic syndrome is central obesity and metabolic disorders, and in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com