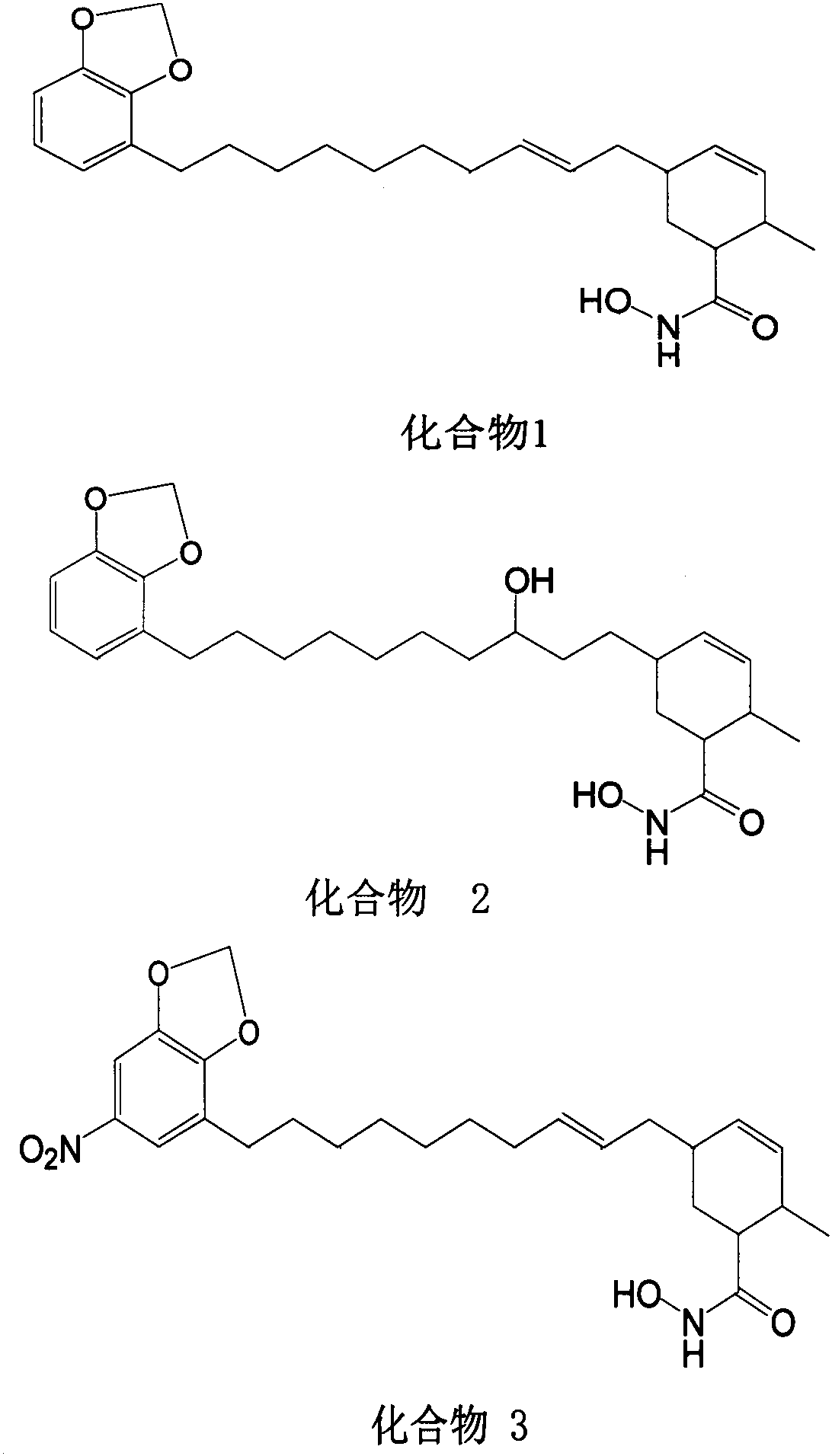
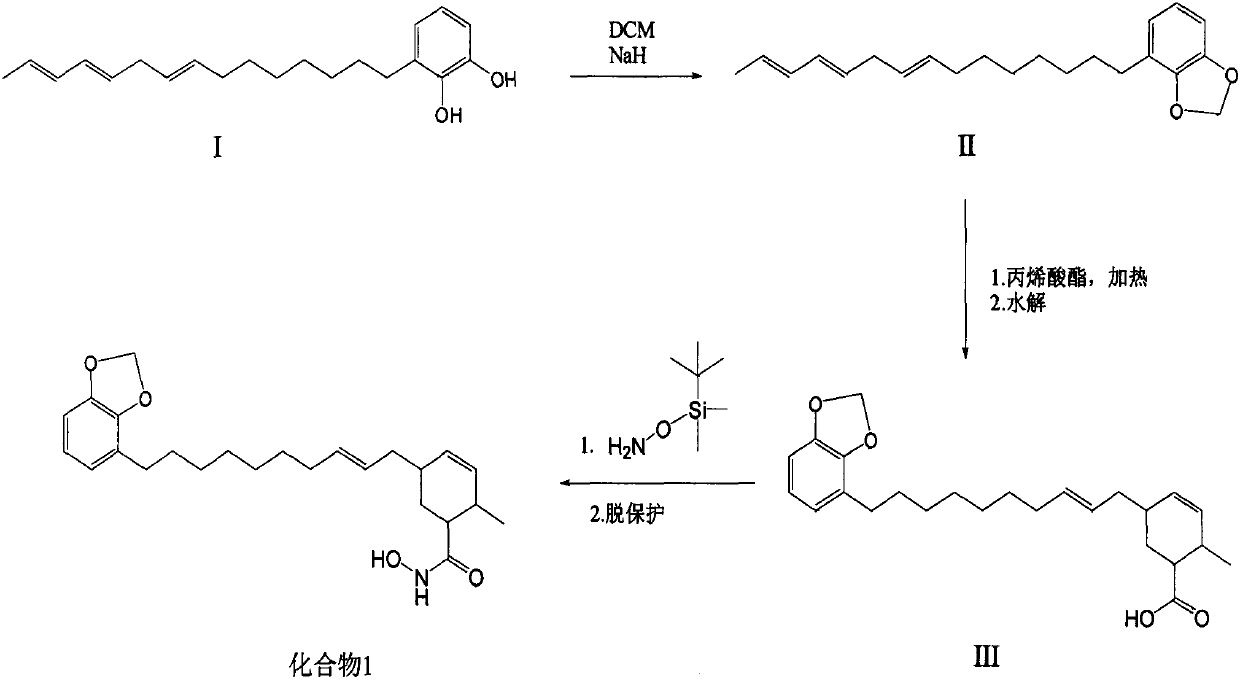
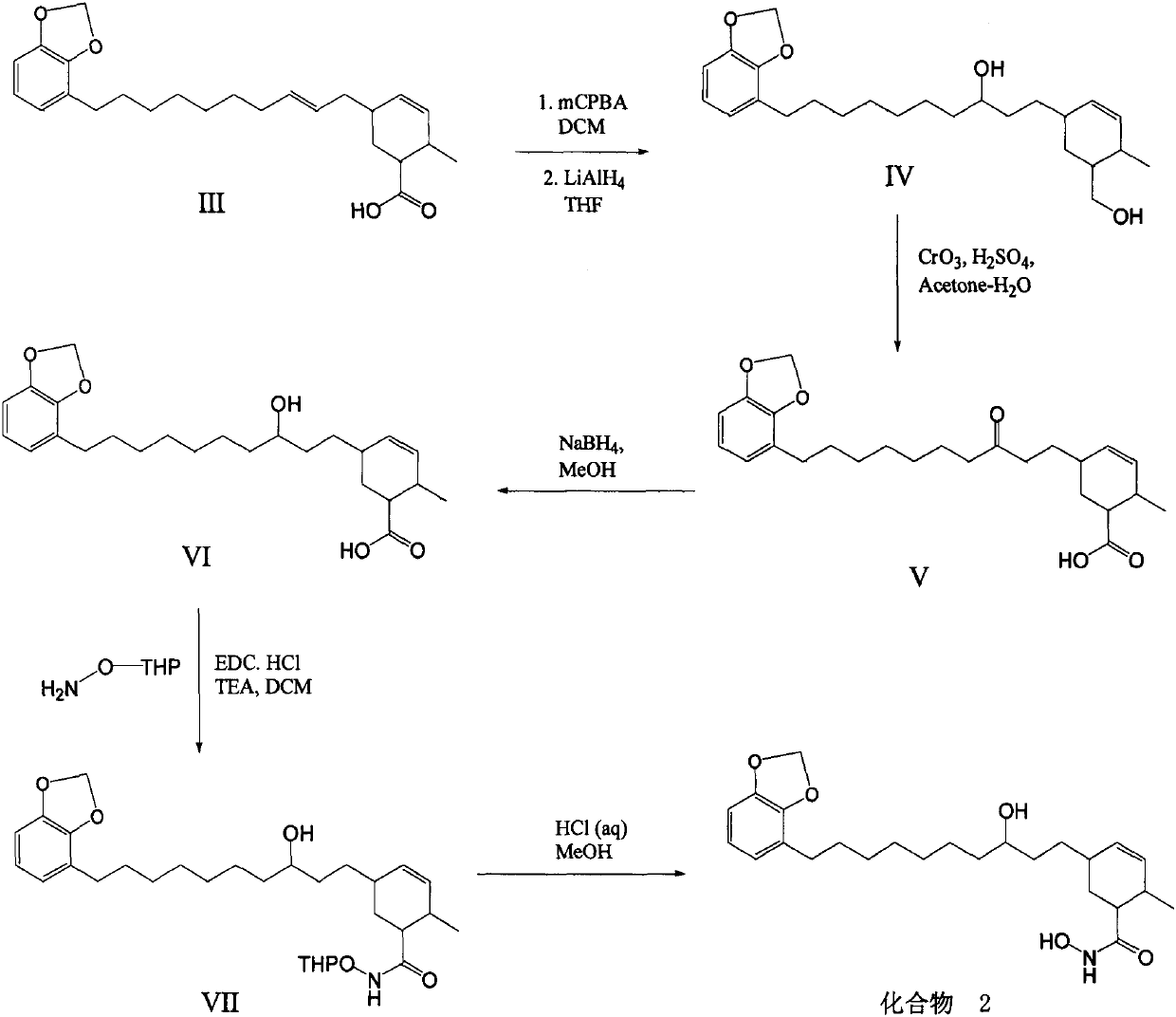
Synthetic method of methylene ether urushiol hydroxamic acid derivatives with (histone deacetylase) HDAC inhibitory activity
A technology of phenol hydroxamic acid and methylene ether, applied in the field of pharmaceutical synthetic chemistry, can solve the problem of lack of zinc ion binding region, a key structural unit of HDAC inhibition, and achieve the effect of wide and easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of target compound 1
[0043] (1) Dissolve 3g of compound I urushiol in 50mL DMF and 50mL DCM, add 0.8g NaH, reflux overnight under the protection of argon, slowly add 100mL water after cooling to room temperature, extract 3 times with DCM, 60mL each time, and combine The extract was washed twice with saturated brine, 90 mL each time, dried over anhydrous sodium sulfate, evaporated to dryness under reduced pressure, column chromatography, eluted with PE, and the eluent was evaporated under reduced pressure to obtain compound II;
[0044] (2) Dissolve 1.2 g of compound II in 2 mL of toluene, add 2 mL of acrylate, heat to reflux for 36 hours under argon protection, evaporate the solvent under reduced pressure, add 10 mL of ethanol and 1.2 g of KOH, heat and reflux for 10 minutes, and evaporate to dryness under reduced pressure Solvent, add 10mL water, extract 3 times with EA, 15mL each time, combine the extracts, dry with anhydrous sodium sulfate, evaporate to drynes...
Embodiment 2
[0056] Synthesis of target compound 1
[0057] (1) Dissolve 4g of compound I urushiol in 60mL DMF and 60mL DCM, add 0.85g NaH, reflux overnight under argon protection, slowly add 100mL water after cooling to room temperature, extract 3 times with DCM, 60mL each time, and combine The extract was washed twice with saturated brine, 100 mL each time, dried with anhydrous sodium sulfate, evaporated to dryness under reduced pressure, column chromatography, eluted with PE, and the eluent was evaporated to dryness under reduced pressure to obtain compound II;
[0058] (2) Dissolve 1.5 g of compound II in 3 mL of toluene, add 3 mL of acrylate, heat to reflux for 36 hours under argon protection, evaporate the solvent under reduced pressure, add 15 mL of ethanol and 1.5 g of KOH, heat to reflux for 10 minutes, and evaporate to dryness under reduced pressure Solvent, add 15mL water, extract 3 times with EA, 15mL each time, combine the extracts, dry with anhydrous sodium sulfate, evaporate to d...
Embodiment 3
[0070] Synthesis of target compound 1
[0071] (1) Dissolve 5g of compound I urushiol in 90mL DMF and 90mL DCM, add 1.2g NaH, reflux overnight under the protection of argon, slowly add 150mL water after cooling to room temperature, extract 3 times with DCM, each 100mL, and combine The extract was washed twice with saturated brine, 100 mL each time, dried with anhydrous sodium sulfate, evaporated to dryness under reduced pressure, column chromatography, eluted with PE, and the eluent was evaporated to dryness under reduced pressure to obtain compound II;
[0072] (2) Dissolve 2.0 g of compound II in 4 mL of toluene, add 4 mL of acrylate, heat to reflux for 36 hours under argon protection, evaporate the solvent under reduced pressure, add 16 mL of ethanol and 1.9 g of KOH, heat to reflux for 10 minutes, and evaporate to dryness under reduced pressure Solvent, add 15mL water, extract 3 times with EA, 15mL each time, combine the extracts, dry with anhydrous sodium sulfate, evaporate to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com