Novel antibody molecule, preparation method thereof and use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0249] Example 1. Construction, expression, purification and characterization of anti-OX40 / PD-L1 bispecific antibody
Embodiment 11
[0250] Example 1.1. Construction of anti-OX40 / PD-L1 bispecific antibody
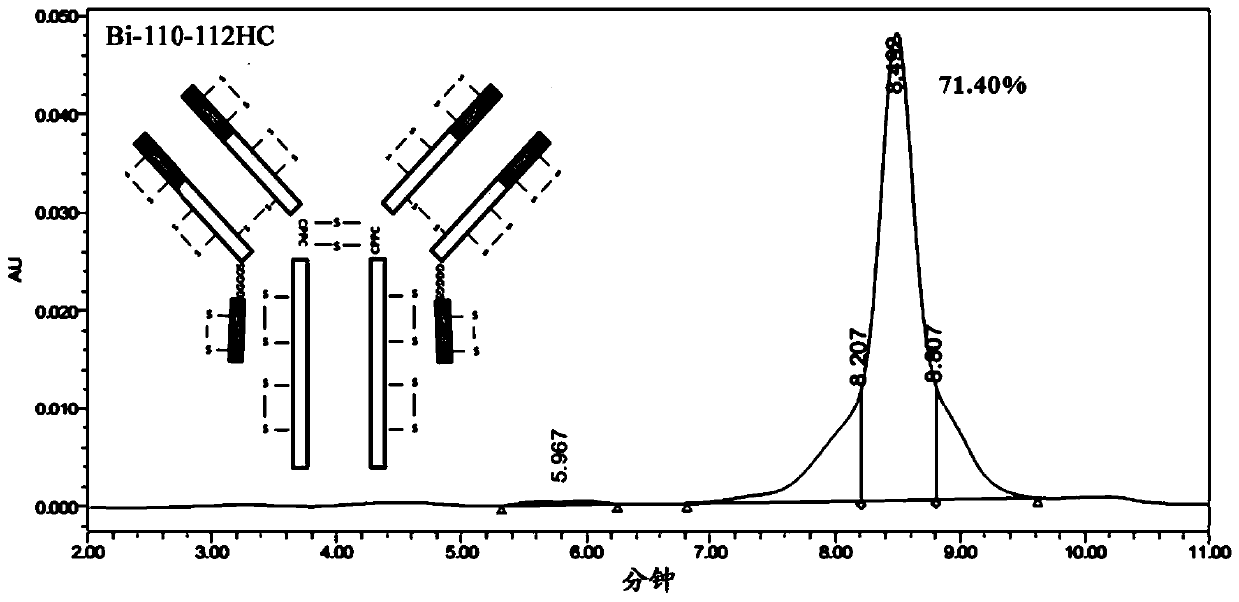
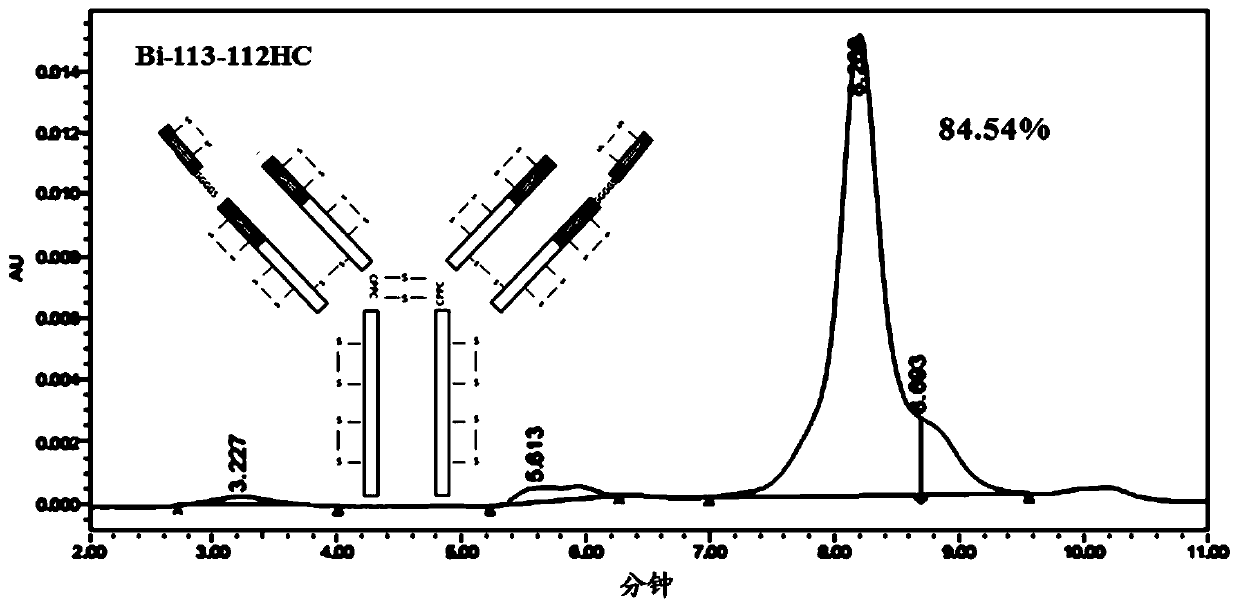
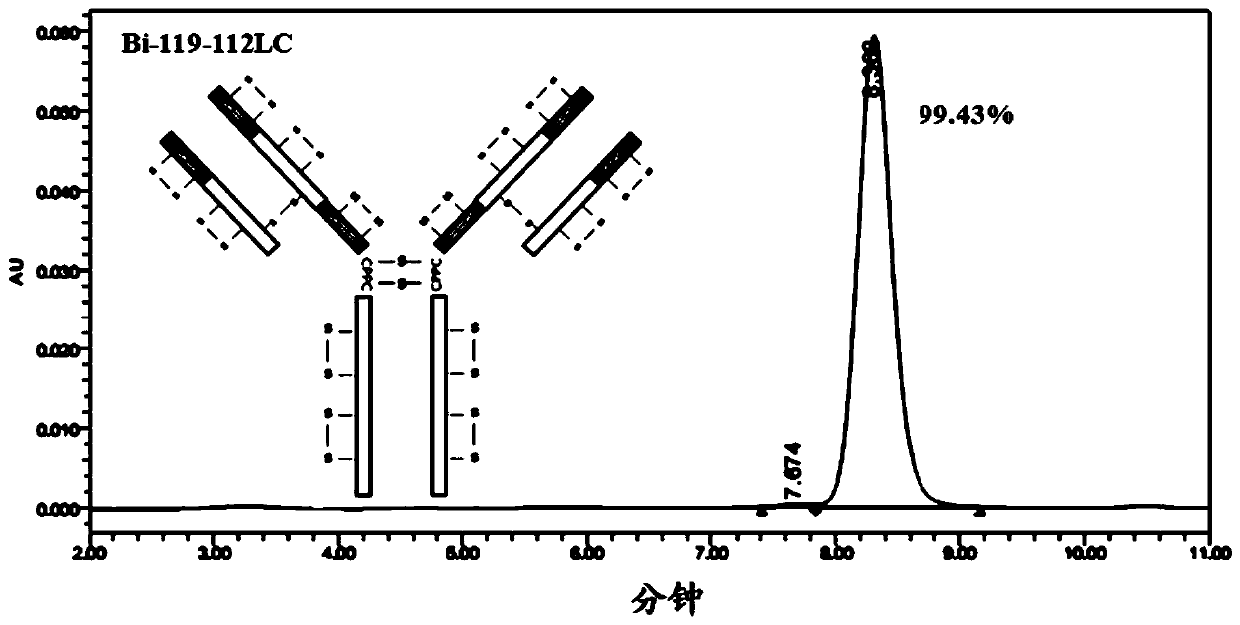
[0251] In this example, four anti-OX40 / PD-L1 bispecific antibodies with different structures were constructed, named respectively (1) bispecific antibody Bi-110-112HC, and its structural schematic diagram is as follows Figure 1A Shown; (2) bispecific antibody Bi-113-112HC, its structural schematic diagram is shown in Figure 1BShown; (3) bispecific antibody Bi-119-112LC, the schematic diagram of its structure is shown in Figure 1C shown; and (4) bispecific antibody Bi-122-112LC, its structural schematic diagram is shown in Figure 1D shown. The four anti-OX40 / PD-L1 bispecific antibodies are described below.
[0252] (1) from Figure 1A It can be seen from the schematic diagram of the structure that the bispecific antibody Bi-110-112HC is composed of four symmetrical polypeptide chains, of which the two polypeptide chains (ie, peptide chain #1 and peptide chain #2) in the left half are from N-terminal...
Embodiment 12
[0256] Example 1.2. Expression, purification and analysis of anti-OX40 / PD-L1 bispecific antibody
[0257] In this example, the nucleotide sequences encoding the peptide chain #1 and peptide chain #2 of the anti-OX40 / PD-L1 bispecific antibody constructed in Example 1.1 were linked into commercially available real The nuclear expression vector pTT5 was expressed and purified in eukaryotic cells to obtain anti-OX40 / PD-L1 bispecific antibodies Bi-110-112HC, Bi-113-112HC, Bi-119-112LC and Bi-122-112LC . The specific operation is as follows.
[0258] Entrusted Suzhou Genewiz Biotechnology Co., Ltd. (Genewiz) to synthesize the coding nucleotides of the above-mentioned peptide chains of bispecific antibodies Bi-110-112HC, Bi-113-112HC, Bi-119-112LC and Bi-122-112LC sequence. The synthesized nucleotide sequences encoding peptide chains were respectively ligated into vector pTT5 by using appropriate restriction enzymes and ligases to obtain recombinant vectors respectively containing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com