Preparation method and application method of kit for fast testing efficacy of antitumor drug
An anti-tumor drug and kit technology, applied in the field of environmental science, can solve the problem that the PDX model cannot be used with immunosuppressants, the evaluation of the efficacy of immunomodulators, the time-consuming drug susceptibility test in the model building cycle, and the tumor formation rate of PDX model transplantation. Low problems, to achieve the effect of rapid and efficient detection, suitable for clinical use, and easy to promote
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
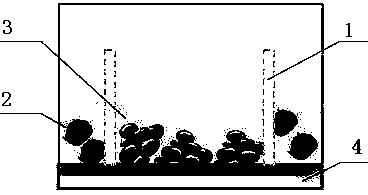
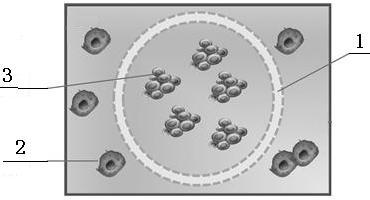
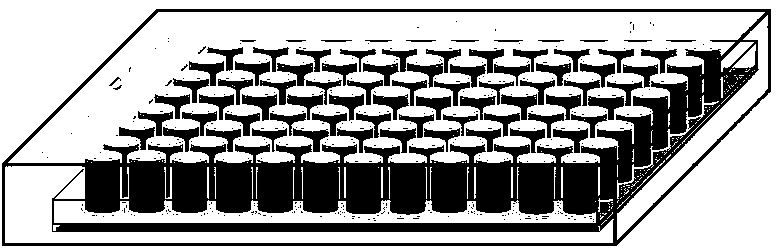
[0046] Such as Figure 1-4 Shown, a kind of preparation method of antineoplastic drug rapid efficacy test kit, the steps are:
[0047] Step 1: Take freshly collected clinical tumor samples or PDX mouse tumor samples in transport preservation tubes, which are filled with liquid nitrogen, and transported to the laboratory for subsequent operations in the shortest possible time.
[0048] The second step, preparation of single cell suspension: remove non-tumor tissue and necrotic tissue from clinical tumor samples or PDX mouse tumor samples in the laboratory, and cut tumor samples into 2mm 3 For small pieces, wash with PBS and collect the tissue pieces, centrifuge at 1000rpm for 5min to remove the supernatant; use type 2 collagenase to incubate the precipitate at 37°C for 2 to 4 hours on a shaker; filter with a 40μm nylon mesh after incubation to remove tissue residues Blocks and cell clusters; centrifuge the filtrate at 1000rpm for 5min to remove the supernatant, resuspend the p...
Embodiment 2
[0068] Such as Figure 1-4 Shown, a kind of preparation method of antineoplastic drug rapid efficacy test kit, the steps are:
[0069] Step 1: Take freshly collected clinical tumor samples or PDX mouse tumor samples in transport preservation tubes, which are filled with liquid nitrogen, and transported to the laboratory for subsequent operations in the shortest possible time.
[0070] The second step, preparation of single cell suspension: remove non-tumor tissue and necrotic tissue from clinical tumor samples or PDX mouse tumor samples in the laboratory, and cut tumor samples into 2mm 3 For small pieces, wash with PBS and collect the tissue pieces, centrifuge at 1000rpm for 5min to remove the supernatant; use type 2 collagenase to incubate the precipitate at 37°C for 2 to 4 hours on a shaker; filter with a 40μm nylon mesh after incubation to remove tissue residues Blocks and cell clusters; centrifuge the filtrate at 1000rpm for 5min to remove the supernatant, resuspend the p...
Embodiment 3
[0091] Such as Figure 1-4 Shown, a kind of preparation method of antineoplastic drug rapid efficacy test kit, the steps are:
[0092] Step 1: Take freshly collected clinical tumor samples or PDX mouse tumor samples in transport preservation tubes, which are filled with liquid nitrogen, and transported to the laboratory for subsequent operations in the shortest possible time.
[0093] The second step, preparation of single cell suspension: remove non-tumor tissue and necrotic tissue from clinical tumor samples or PDX mouse tumor samples in the laboratory, and cut tumor samples into 2mm 3 For small pieces, wash with PBS and collect the tissue pieces, centrifuge at 1000rpm for 5min to remove the supernatant; use type 2 collagenase to incubate the precipitate at 37°C for 2 to 4 hours on a shaker; filter with a 40μm nylon mesh after incubation to remove tissue residues Blocks and cell clusters; centrifuge the filtrate at 1000rpm for 5min to remove the supernatant, resuspend the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com