Method for identifying L-phenylalanine
A technology for phenylalanine and identification methods, applied in chemical instruments and methods, instruments, analytical materials, etc., can solve the problem of low specificity, and achieve the effect of simple detection process and equipment, low cost, and simple identification methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: A method for identifying L-phenylalanine, the steps are as follows:
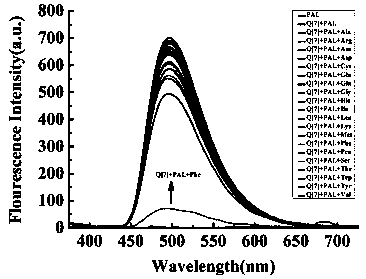
[0043] 1) Preparation of standard probe solution: Dissolve seven-membered melon rings in water to obtain solution A; dissolve patinol in water to obtain solution B; mix solution A with solution B to control seven-membered melon rings and patinol The molar ratio is 1:1, react at room temperature to obtain a probe solution, in which the molecular formula of the probe is C 42 h 42 N 28 o 14 @C 21 h 22 NO 4 , the probe model diagram is attached image 3 As indicated, the probe solution was diluted with neutral water to a concentration of 2.0×10 -5 mol / L, to obtain the standard probe solution;
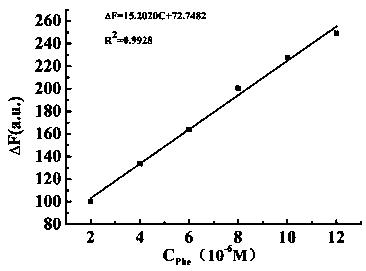
[0044] 2) L-phenylalanine recognition: Add the solution to be tested dropwise into the standard probe solution, let it stand for 10s, then measure the fluorescence spectrum at a fixed excitation wavelength of 342nm, and draw the fluorescence intensity at the excited laser wavelength When the ...
Embodiment 2
[0045] Embodiment 2: A method for identifying L-phenylalanine, the steps are as follows:
[0046] 1) Preparation of standard probe solution: Dissolve seven-membered melon rings in water to obtain solution A; dissolve patinol in water to obtain solution B; mix solution A with solution B to control seven-membered melon rings and patinol The molar ratio is 1:0.5, react at room temperature to obtain a probe solution, dilute the probe solution with neutral water to a concentration of 2.0×10 -5 mol / L, to obtain the standard probe solution;
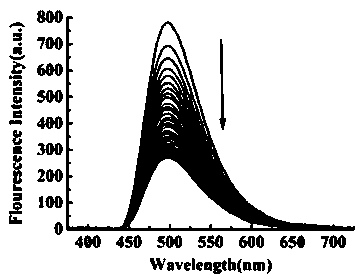
[0047] 2) L-Phenylalanine recognition: Add the solution to be tested dropwise into the standard probe solution, let it stand for 5s, then measure the fluorescence spectrum at a fixed excitation wavelength of 342nm, and draw the fluorescence intensity at the excited laser wavelength When the fluorescence intensity at 493nm is quenched, it indicates that the solution to be tested contains L-phenylalanine, otherwise it does not.
Embodiment 3
[0048] Embodiment 3: A method for identifying L-phenylalanine, the steps are as follows:
[0049] 1) Preparation of standard probe solution: Dissolve seven-membered melon rings in water to obtain solution A; dissolve patinol in water to obtain solution B; mix solution A with solution B to control seven-membered melon rings and patinol The molar ratio is 1: 2, react at room temperature to obtain a probe solution, dilute the probe solution with neutral water to a concentration of 2.0×10 -5 mol / L, to obtain the standard probe solution;
[0050] 2) L-phenylalanine recognition: Add the solution to be tested dropwise into the standard probe solution, let it stand for 15s, then measure the fluorescence spectrum at a fixed excitation wavelength of 342nm, and draw the fluorescence intensity at the excited laser wavelength When the fluorescence intensity at 493nm is quenched, it indicates that the solution to be tested contains L-phenylalanine, otherwise it does not.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com