Phosphaplatin compounds as therapeutic agents for treatment of bone blood cancers
A compound and blood cancer technology, applied in the direction of active ingredients of phosphorus compounds, drug combination, drug delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
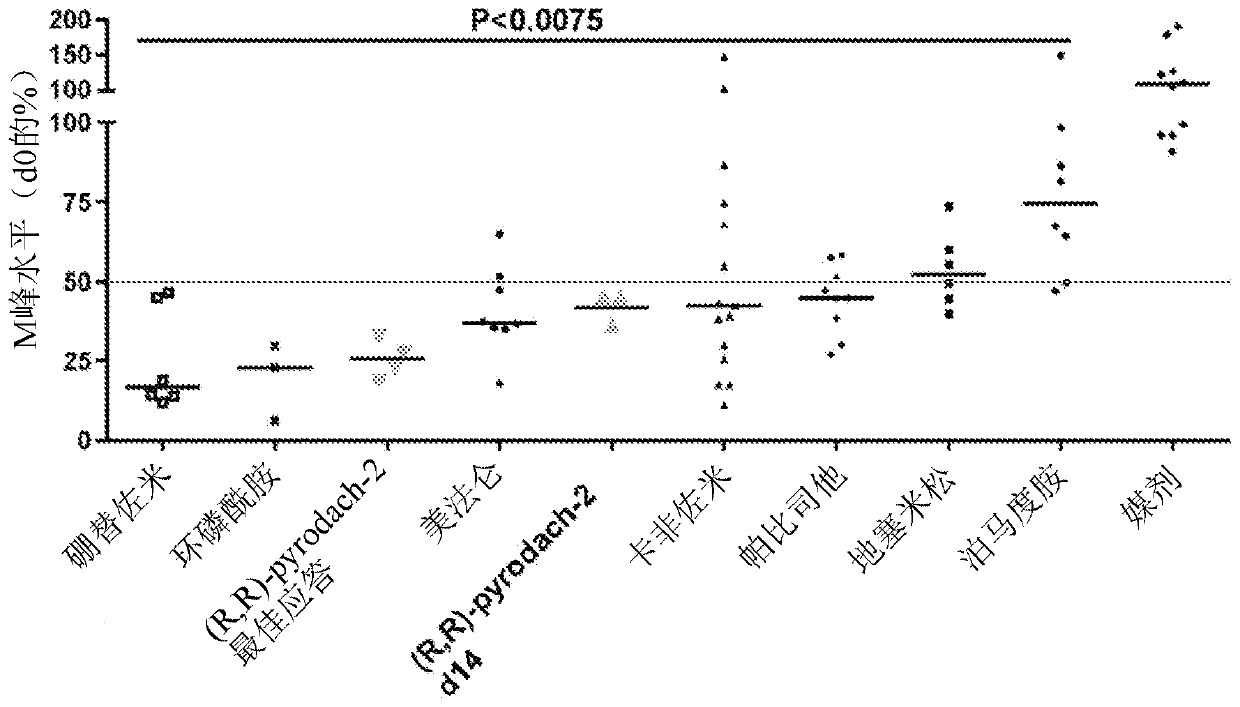
[0080] Determination of R,R-pyrodach-2 against multiple myeloma cell lines
[0081] The compound trans-(R,R)-1,2-cyclohexanediamine-(dihydropyrophosphate)platinum(II) (“R,R”) was tested on two multiple myeloma cell lines RPMI 8226 and MM1R -pyrodach-2"). IC50 values of 2.90 uM and 2.78 uM were found for R,R-pyrodach-2 against the cell lines RPMI 8226 and MM1R, respectively, indicating the potency and activity of the compounds.
example 2
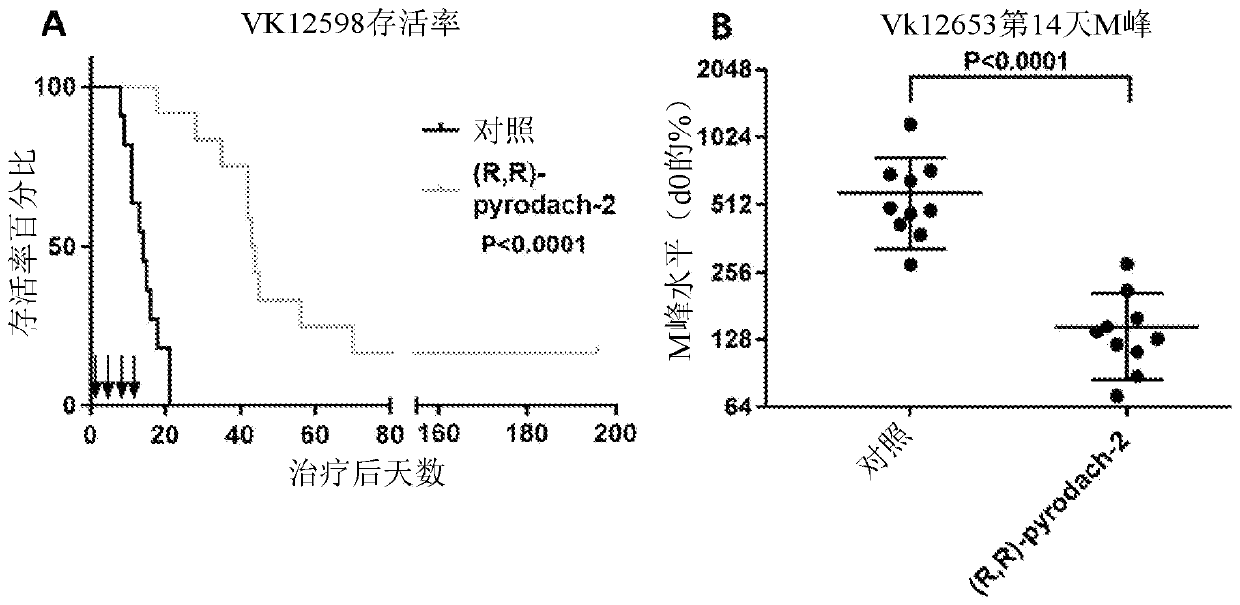
[0083] Detection of R,R-pyrodach-2 in Multiple Myeloma Mouse Model
[0084] background
[0085] Trans-pododach-2 compounds were tested on multiple myeloma mouse models, specifically Vk*MYC mice, which were reported to be reliable predictors of clinical activity of drugs in untreated and relapsed MM Preclinical models (Chesi, M. et al., "Cancer Cell", 2008, 23, 167-180; Chesi, M. et al., "Blood", 2012, 120(2): 376-385 ). Additionally, trans-pyrodach-2 compounds were detected in mice transplanted with the more aggressive bortezomib-resistant Vk12598 cell line and the multidrug-resistant Vk12653 cell line, both of which were derived from Vk*MYC mice were generated.
[0086] Materials and methods
[0087] Neonatal Vk*MYC mice were grown to one year or more, and peak M levels were monitored. After the concentration of the major M peak reaches a level between about 10 g / L and about 70 g / L (estimated by densitometry, comparing M peak to albumin), mice are candidates for initia...
example 3
[0095] Determination of Platinum Distribution in R,R-pyrodach-2 Treated Mice
[0096] Materials and methods
[0097] Five CD-1 mice were given (R,R)- pyrodach -2, and vehicle (phosphate buffered saline) was administered to another mouse. After administration, the (R,R)- pyrodach -2 treated mice were euthanized, and control mice were euthanized 45 minutes after dosing. Subsequently, the cadavers were snap-frozen at -70°C. Slides of whole-body sagittal cross-sections of control 45-minute and 24-hour mice were prepared. Then, the slide was scanned using Laser Ablation Inductively Coupled Plasma Mass Spectrometry (Laser Ablation Inductively Coupled Plasma Mass Spectrometry, LA-ICP-MS) to obtain the Pt((R,R)- pyrodach -2) the concentration of atomic components). Additional slides were prepared from adjacent locations and stained with hematoxylin & eosin (H&E) to map Pt signals to different organs and tissues.
[0098] result
[0099] It was observed that in processed s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com