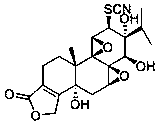
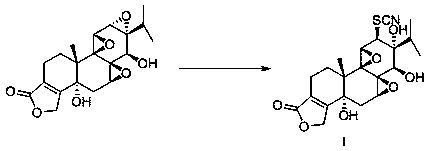
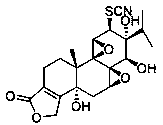
(5R)-5-hydroxyl triptolide derivative and preparation method and application thereof
A technology of lactone alcohol and derivatives, applied in the field of medicine, can solve problems such as toxicity reduction, and achieve the effect of reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (5R)-5-Hydroxytripterygium thiocyanatolactoneol
[0022]
[0023] Preparation method: Add (5R)-5-hydroxytriptolide (126mg, 0.33mmol) into 15ml tert-butanol, stir at 80°C to dissolve (5R)-5-hydroxytriptolide. Ammonium thiocyanate (376 mg, 4.95 mmol) was then added to the reaction solution. Then the reaction solution was refluxed for reaction, and TLC was used to detect the reaction progress. After the reaction, 40ml of EtOAc was added to the reaction solution. The organic layer was washed three times with saturated NaCl aqueous solution, dried over anhydrous sodium sulfate, and filtered. Concentrate under reduced pressure, water bath temperature 55 ° C, to obtain a white solid, column chromatography, silica gel (40g, 200~300 mesh), with CH 2 Cl 2 / MeOH (100:1) was eluted, and the fractions were collected and dried in vacuo to obtain 82 mg of the product with a yield of 57%. mp: 248-250°C. Kedd's reagent was purple. 1 H NMR (400 MHz, DMSO- d 6 ) δ 6.35 (1s, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com