Transgenic animal expressing hla-a24 and utilization thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
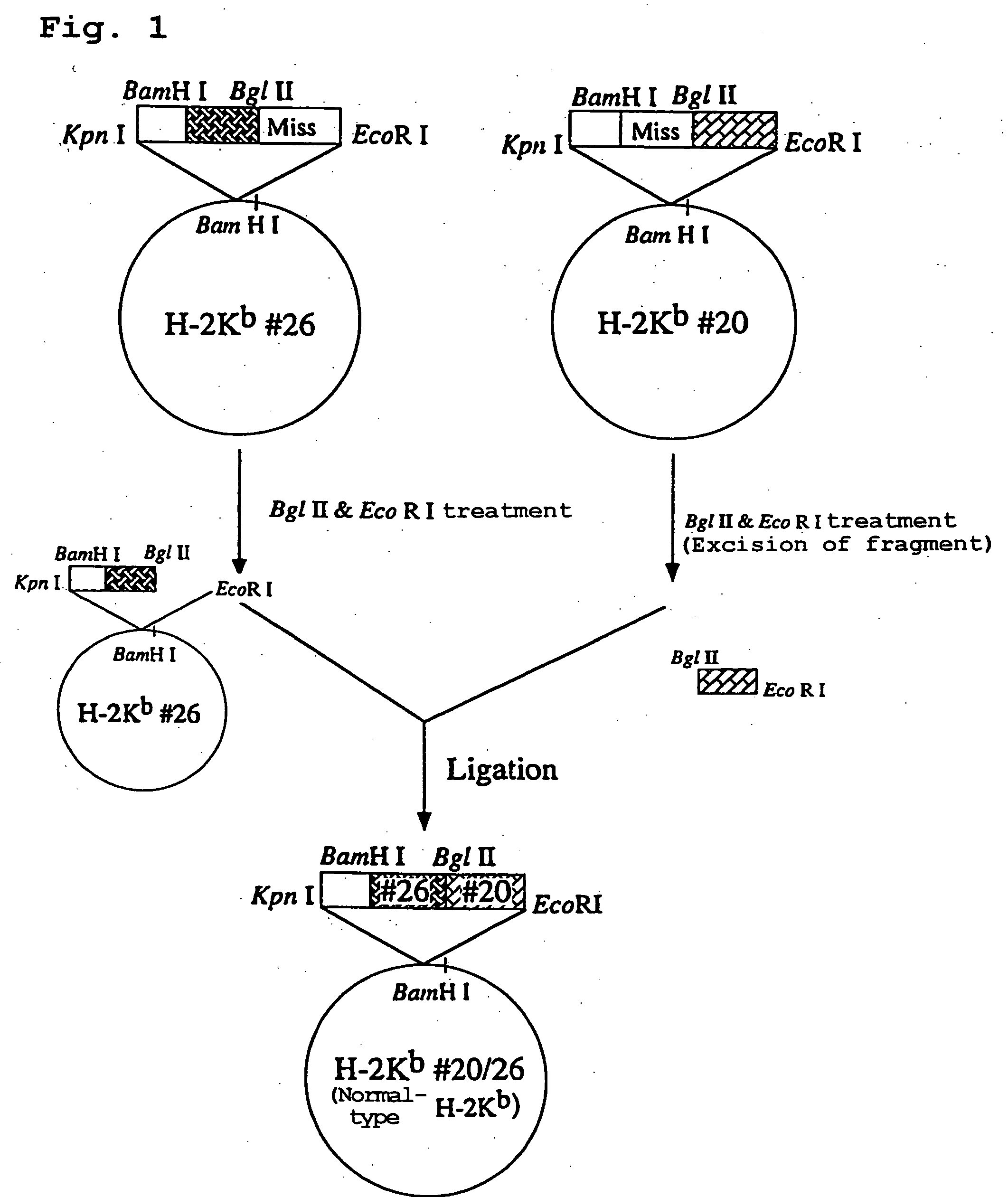
Cloning of H-2K.sup.b Genomic DNA Fragment
[0169] (1) Cloning of H-2K.sup.b Genomic DNA Fragment
[0170] Mouse tumor cell line EL4 (ATCC T1B-39) was cultured, and mouse genomic DNA was purified and used in the PCR cloning. Purification of DNA was carried out using TaKaRa LA Taq.TM. (Takara Shuzo) suited for the amplification of long-chain DNA as per the attached protocol. The GenBank database was then searched for H-2K.sup.b gene needed for the construction of chimeric HLA gene, which revealed that said gene was divided in two segments registered under the Accession Nos. v00746 and v00747. As v00746, the upstream 1594 bp region of H-2K.sup.b down to midstream of intron 3 was registered and, as v00747, the downstream 1837 bp region of H-2K.sup.b down to midstream of intron 7 was registered. Because there was no BamHI restriction site in intron 3, which is divided and registered as v00746 and v00747, the H-2K.sup.b gene registered at the database was thought to have incomplete length.
[01...
example 3
Construction of Chimera Genomic DNA (HLA-A2402 / K.sup.b DNA)
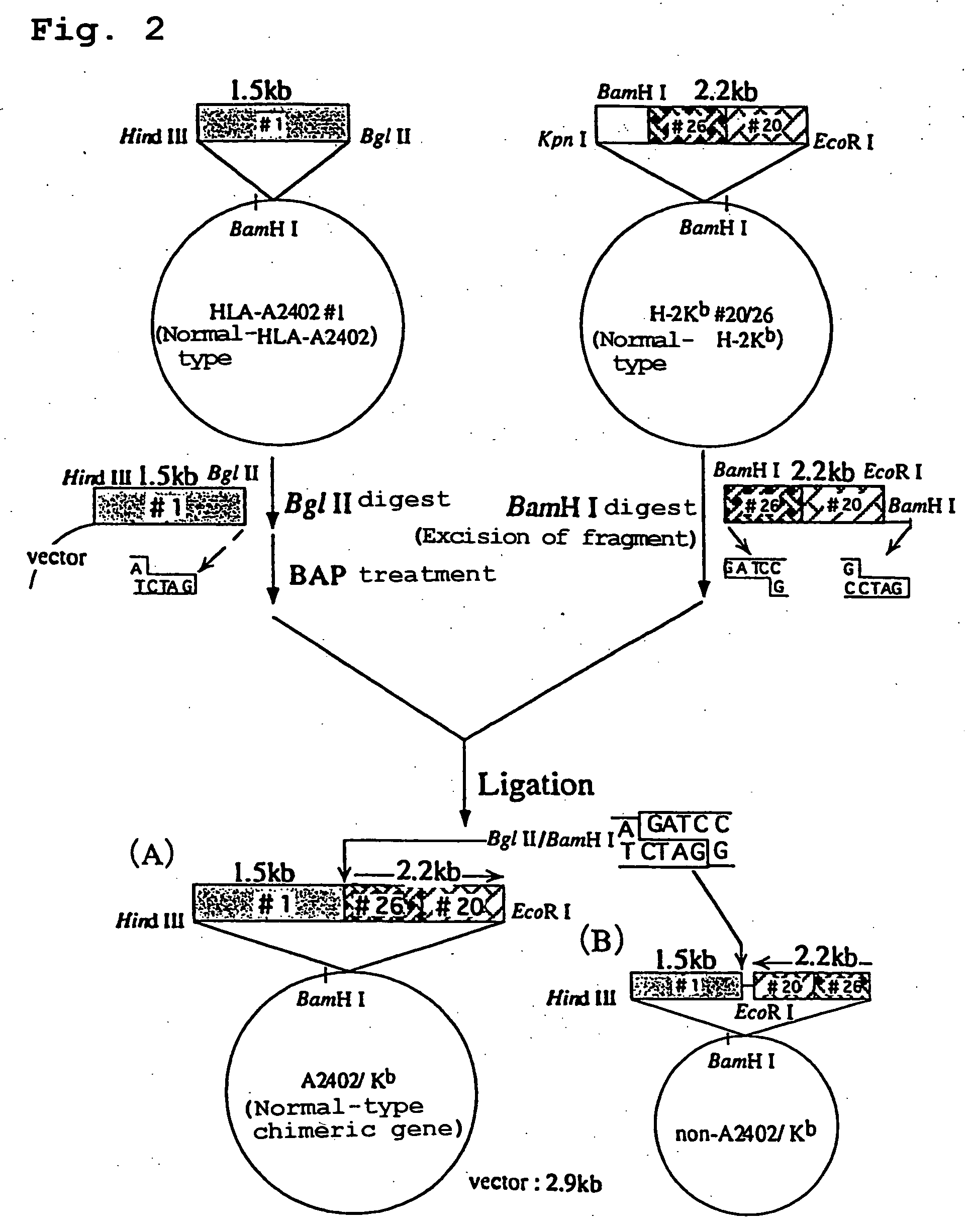
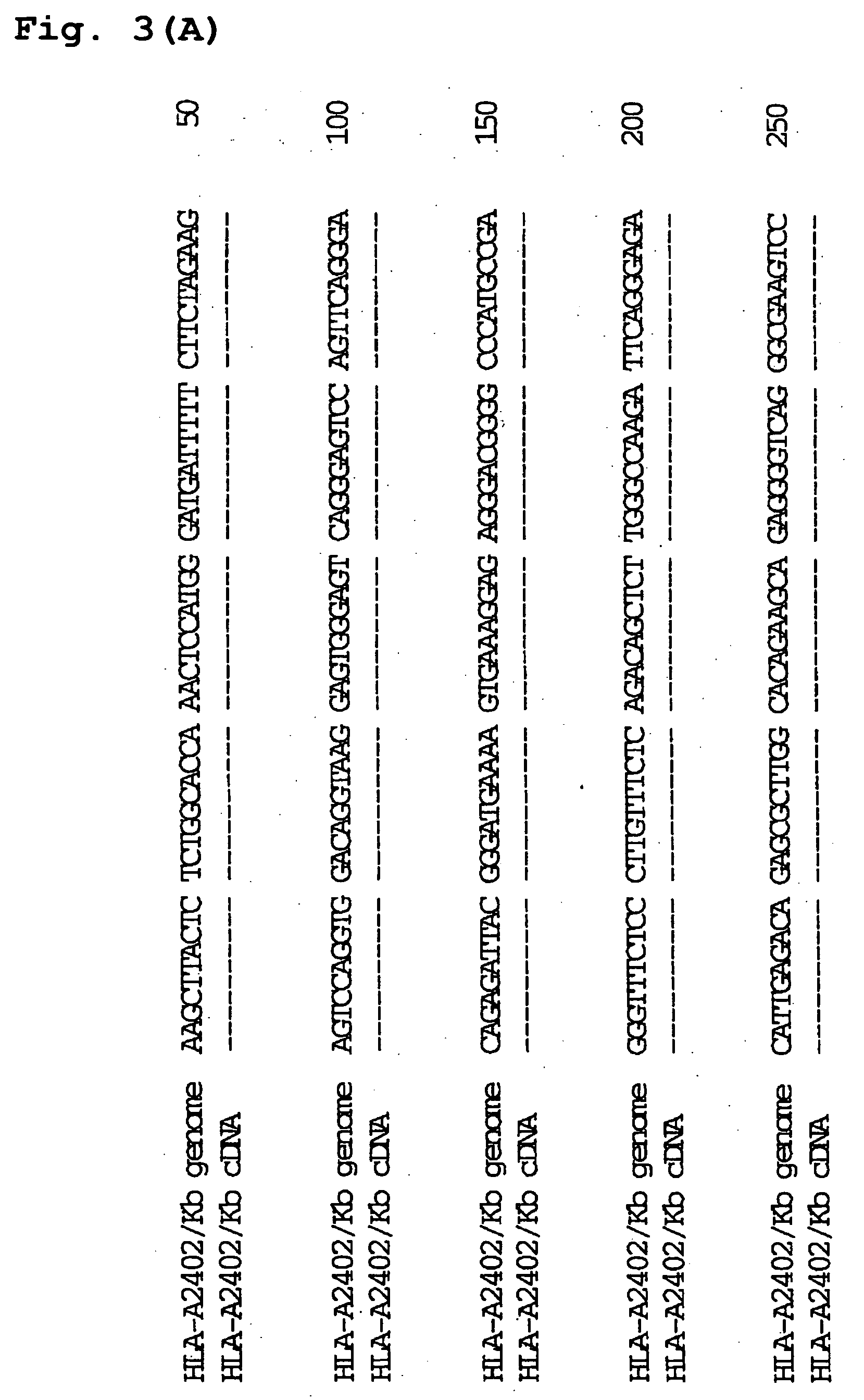
[0187] The Plasmid HLA-A2402#1 containing HLA-A2402 genomic DNA obtained in Example 1 was cleaved at BglII restriction site and the plasmid H-2K.sup.b#20 / 26 containing H-2K.sup.b genomic DNA obtained in Example 2 was cleaved at BamHI restriction site, and the resultant fragments were ligated to give a recombinant plasmid. The schematic construction is shown in FIG. 2. The recombinant plasmid was introduced into E. coli JM109 (Toyobo) by heat shock method at 42.degree. C., and white colonies of E. coli to which the recombinant plasmid has been introduced were selected on ampicillin-containing LB agar medium coated with X-Gal and IPTG to obtain the transformants. Ten transformants were incubated overnight in LB medium containing ampicillin (3 ml). The plasmid clone contained in each transformant was purified and subjected to sequence analysis in a similar manner to the above. As a result, it was revealed that three transforman...
example 4
Splicing Analysis of Chimera Genomic DNA
[0188] Mouse tumor cell line EL4 was transfected with the constructed chimeric HLA gene (HLA-A2402 / K.sup.b gene) with Electro Gene Transfer GTE-10 (Shimadzu) as per the attached protocol. Two days later, total RNA was purified from transfected EL4 cells and un-transfected EL4 cells (control) by using ISOGEN (Nippon Gene) as per the attached protocol. Reverse transcription was performed using SuperScript Choice System (GIBCO BRL) as per the attached protocol using Oligo(dT).sub.12-18 and a part of said RNA as a template to synthesize cDNA. In addition, chimera gene was specifically amplified by PCR using Native Pfu DNA Polymerase (Stratagene) and a part of said cDNA as a template.
[0189] PCR was conducted using an upstream primer Chimera-F2:
[0190] 5'-CGA ACC CTC GTC CTG CTA CTC TC-3' (23 mer, SEQ ID NO:10), which is encoded in exon 1 of HLA-A2402 gene and has low homology with H-2K.sup.b gene, and a downstream primer Chimera-R2:
[0191] 5'-AGC ATA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com