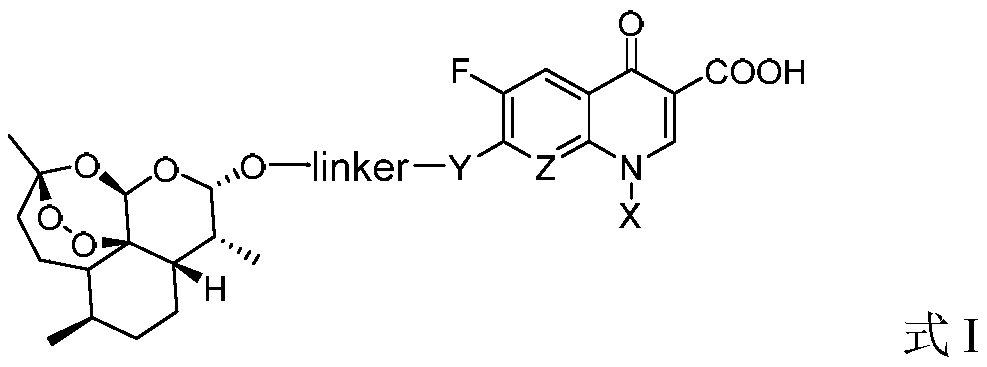
Application of dihydroartemisinin and quinolone conjugate in preparation of Wnt signaling pathway agonist
A technology of quinolone conjugates and dihydroartemisinin, which is applied in the direction of drug combinations, medical preparations containing active ingredients, organic active ingredients, etc., to achieve the effect of broadening the use of pharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1. Preparation of target compound
[0036] 1. Synthesis of the target compound TM1 series
[0037] The target compounds TM1 series (TM1-1~TM1-12) were prepared according to the method described in Chinese patent 104418864B (conjugates of dihydroartemisinin and quinolones and their preparation methods and applications).
[0038]
[0039]
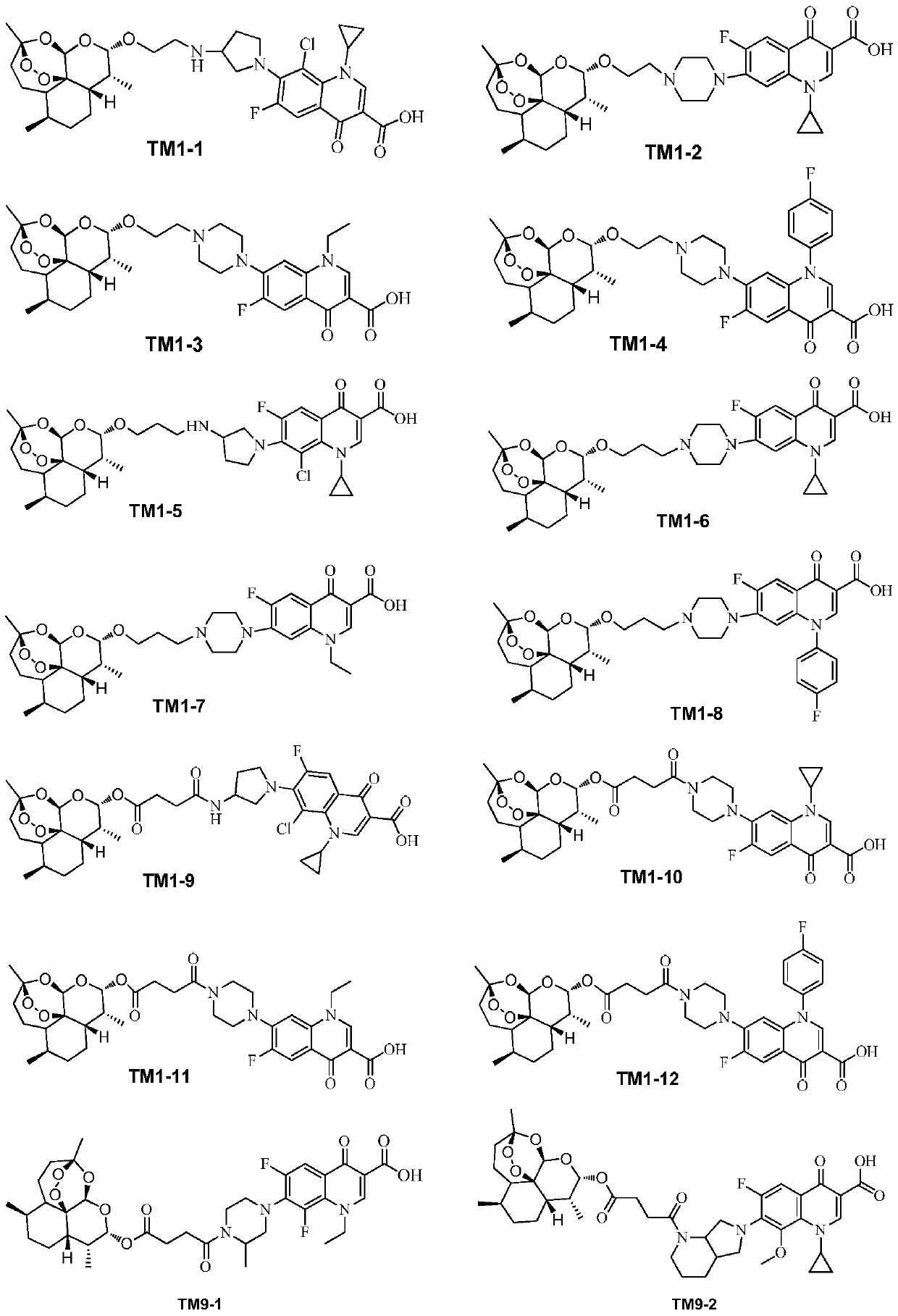
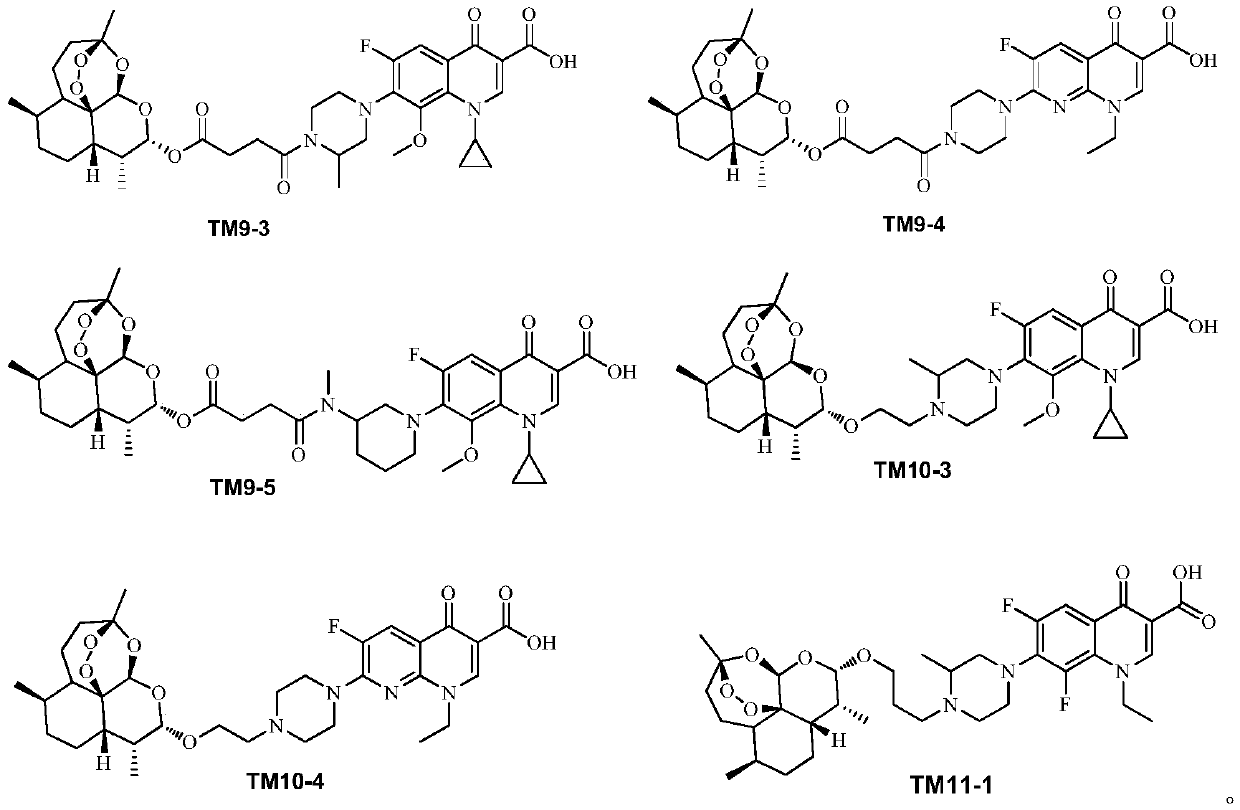
[0040] 2. Synthesis of the target compound TM9 series
[0041] 1) Synthesis of intermediate IM3
[0042] The intermediate IM3 was prepared according to the method described in Chinese patent 104418864B (conjugates of dihydroartemisinin and quinolones and its preparation method and application).
[0043] 2) Synthesis of target compound TM9 series
[0044]
[0045] Add IM3 (1mmol) and 3mL of dichloromethane (DCM) into a 100mL reaction flask, stir at -10°C to 0°C, and partially dissolve, then add N,N'-diisopropylethylamine (DIPEA, 1.5mmol) in sequence , Pivaloyl chloride (1.5mmol), -10 ℃ ~ 0 ℃ continue to stir the ...
Embodiment 2
[0079] Example 2. The Wnt signaling pathway agonistic activity test of the target compound
[0080] The Wnt signaling pathway activating activity of the target compound was tested by the Open Innovation Drug Discovery (OIDD) program of Eli Lilly and Company. Firstly, a single-concentration primary screening (Primary SP) was performed, and then a multi-concentration test (Primary CRC) was performed on the potential molecules screened out initially. . The test results of the Wnt signaling pathway agonist activity of some compounds are shown in Table 3 and Table 4.
[0081] Table 3 Wnt signaling pathway agonistic activity test results-1
[0082]
[0083]Table 1 tested the activity of the target compound TM1 series at different concentrations to promote the expression of β-catenin protein in the nucleus in mouse myoblast C2C12 (cultured in Wnt-conditioned medium), and the target compound TM1 series promoted C2C12 cells under Wnt3a stimulation. The median effect concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com