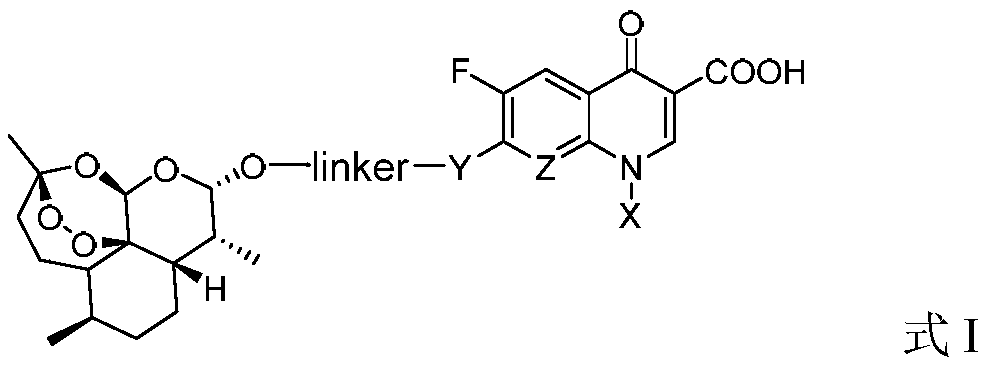
Application of dihydroartemisinin and carbostyril conjugates to preparation of medicines for reducing blood lipid
A quinolone conjugate, dihydroartemisinin technology, applied in the directions of drug combination, pharmaceutical formula, medical preparation containing active ingredients, etc., to achieve the effect of broadening the use of pharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1. Preparation of target compound
[0035] 1. Synthesis of the target compound TM1 series
[0036] The target compounds TM1 series (TM1-1~TM1-12) were prepared according to the method described in Chinese patent 104418864B (conjugates of dihydroartemisinin and quinolones and their preparation methods and applications).
[0037]
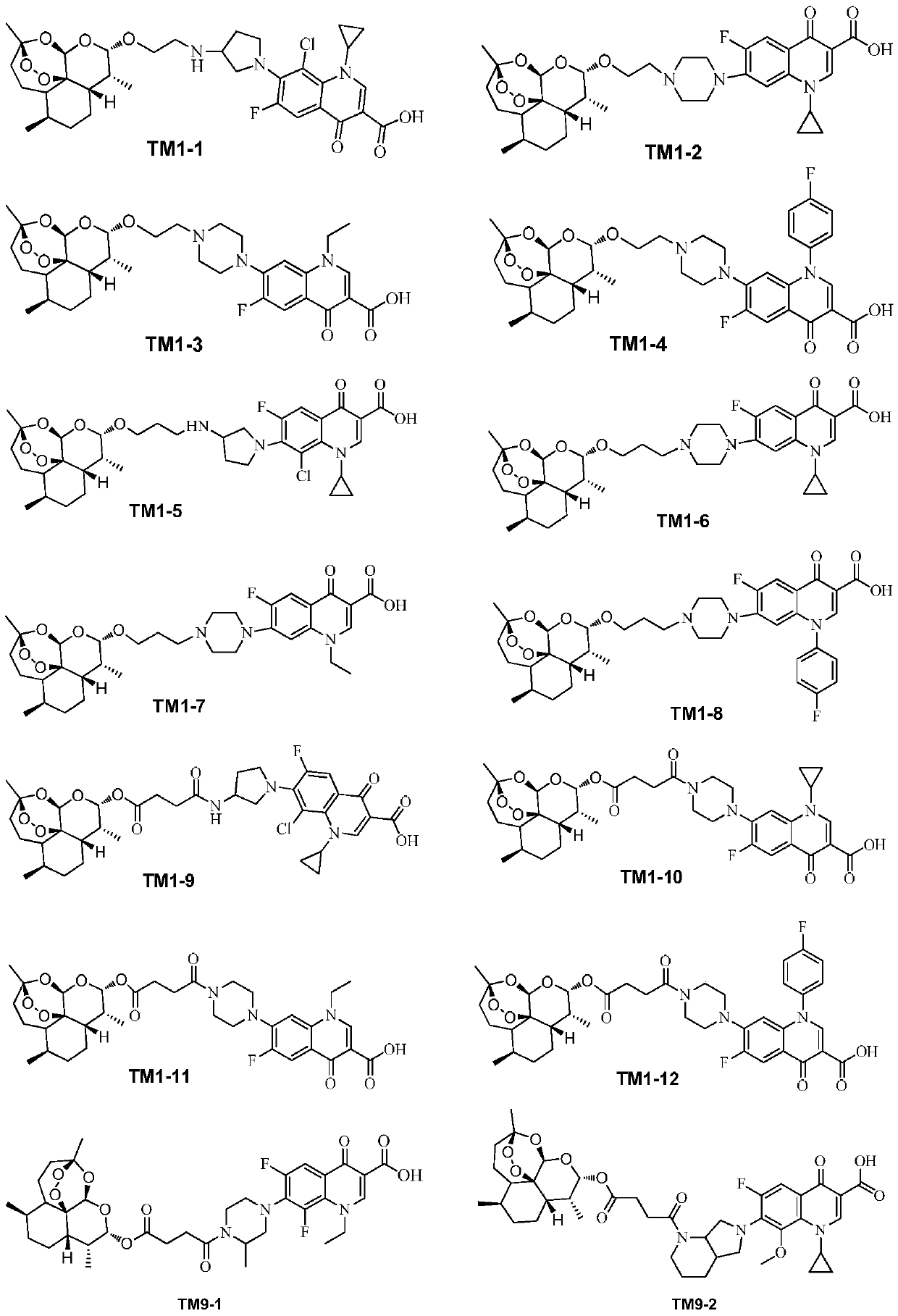
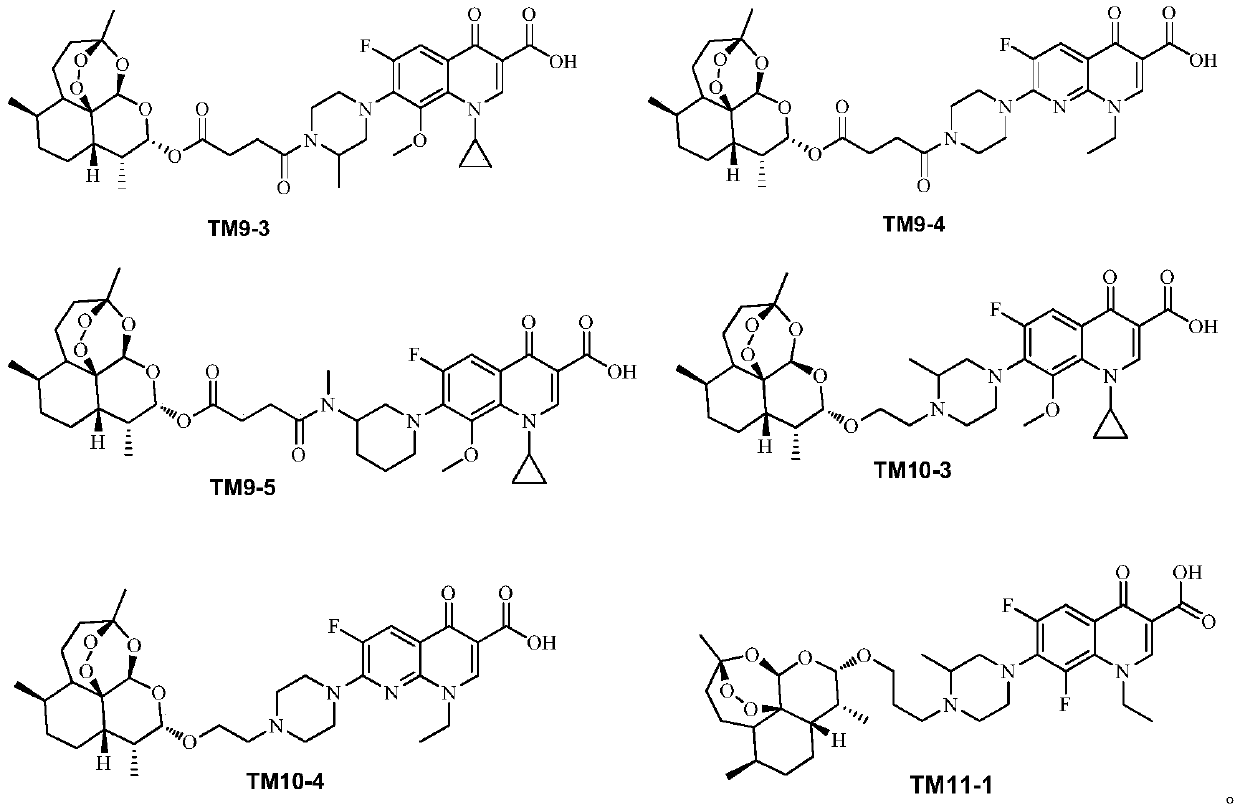
[0038] 2. Synthesis of the target compound TM9 series
[0039] 1) Synthesis of intermediate IM3
[0040] The intermediate IM3 was prepared according to the method described in Chinese patent 104418864B (conjugates of dihydroartemisinin and quinolones and its preparation method and application).
[0041] 2) Synthesis of target compound TM9 series
[0042]
[0043] Add IM3 (1mmol) and 3mL of dichloromethane (DCM) into a 100mL reaction flask, stir at -10°C to 0°C, and partially dissolve, then add N,N'-diisopropylethylamine (DIPEA, 1.5mmol) in sequence , Pivaloyl chloride (1.5mmol), -10 ℃ ~ 0 ℃ continue to stir the reaction for...
Embodiment 2
[0076] Example 2. PCSK9 inhibitory activity test of target compound
[0077] The PCSK9 inhibitory activity of the target compound was tested by Eli Lilly's Open Innovation Drug Discovery (OIDD) program. First, a single-concentration primary screening (Primary SP) was performed, and then a multi-concentration test (Primary CRC) was performed on the potential molecules screened out initially. The test results of PCSK9 inhibitory activity of some compounds are shown in Table 3 and Table 4.
[0078] Table 3PCSK9Inhibition (Eff-1) activity test result
[0079]
[0080] Table 3 tested the inhibition rate of the target compound TM1 series on the secretion of PCSK9 from human liver cancer cells HepG2 and the toxicity to HepG2 cells. The results of the Primary SP test showed that the target compound TM1 series could inhibit the secretion of PCSK9 from HepG2 cells. At a test concentration of 20 μM, the inhibition rate of 10 compounds exceeded 90%, and the inhibition rate of 6 compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com