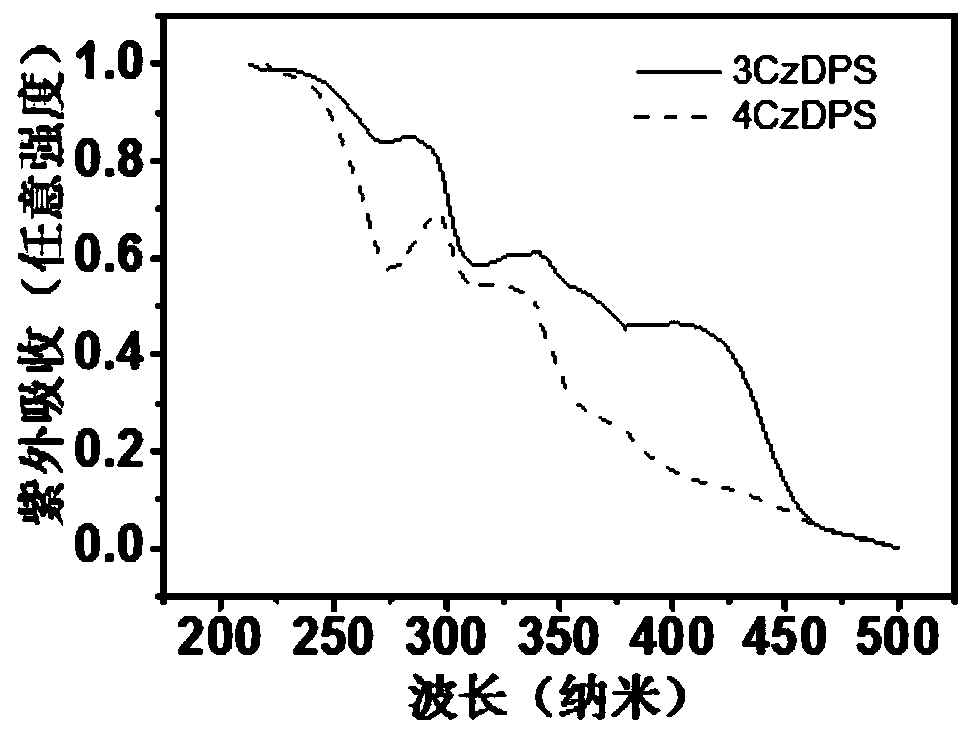
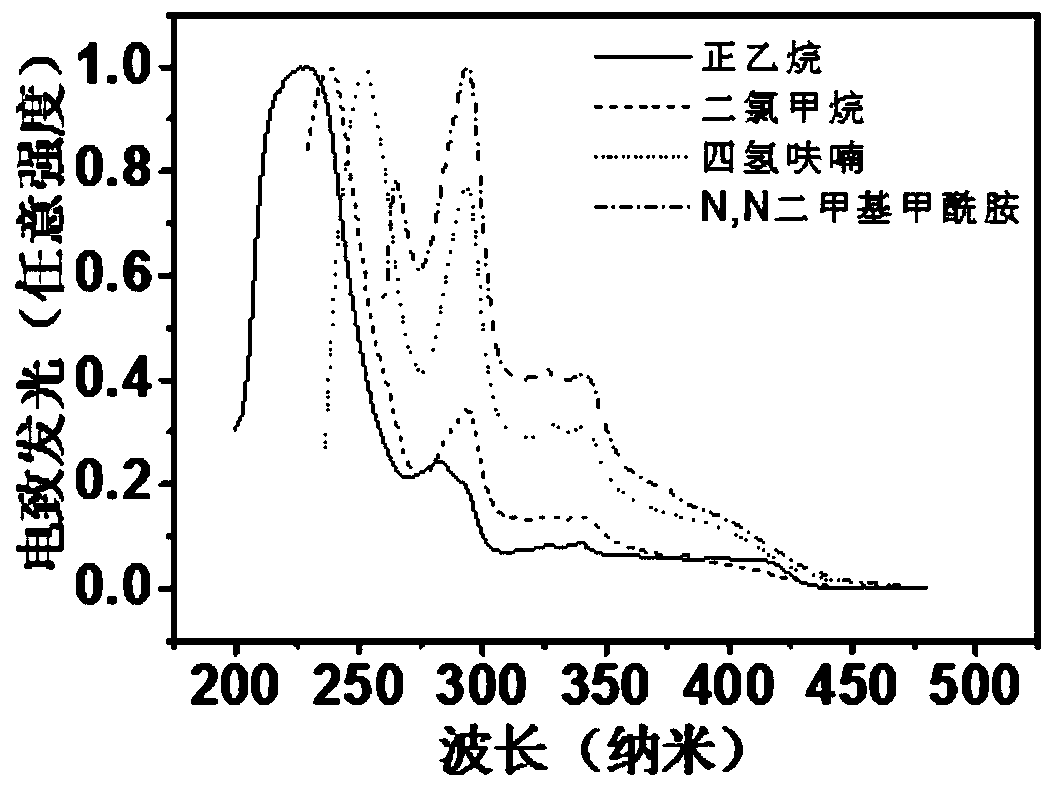
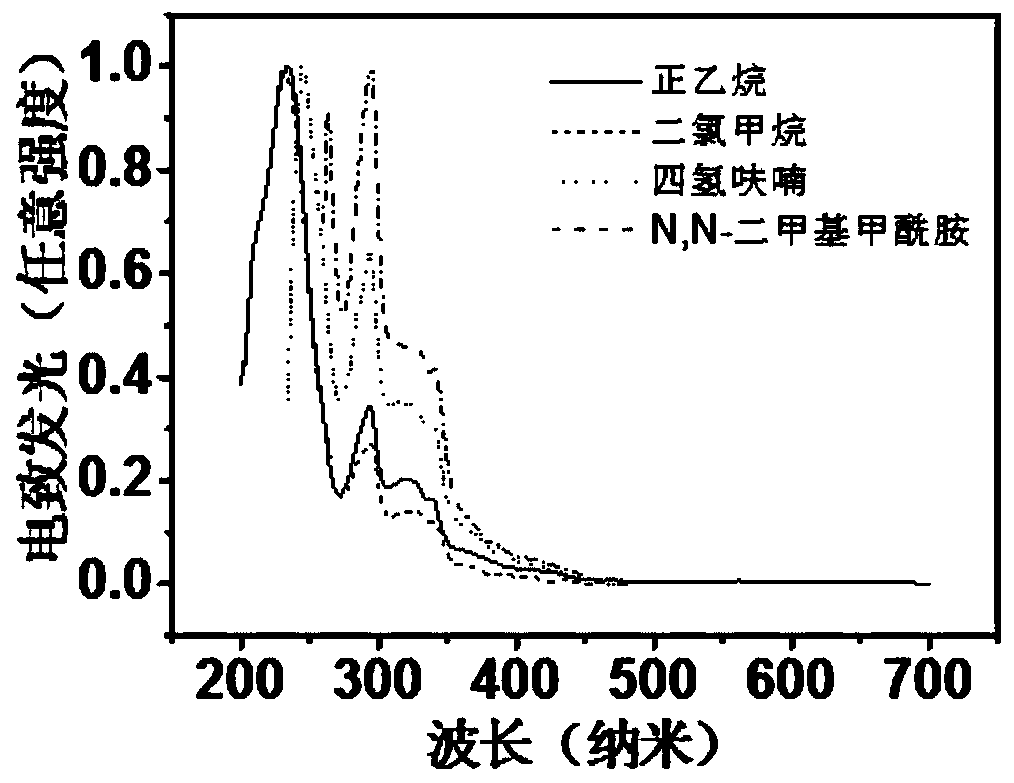
D-A type TADF material, preparation method and application thereof
A technology of D-A and linking method, which is applied in the direction of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problems of poor film formation and difficult device preparation, and achieve low synthesis cost, strong operability, The effect of good film formation and shape stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] The invention discloses a preparation method of a D-A type TADF material, the method comprising the following steps:
[0068] S1: Synthesis of 3BrDPS;
[0069] S2: Synthesizing the first TADF material 3CzDPS on the basis of the step S1;
[0070] S3: Synthesis of 4BrDPS
[0071] S4: Synthesizing the second TADF material 4CzDPS on the basis of the step S3.
Embodiment 1
[0073] Synthesis of 3CzDPS
[0074] S10: Preparation of 1-((3,5-dibromophenylsulfone)-3-bromobenzene (3BrDPS)
[0075] Take a 50mL three-neck flask, dry it thoroughly and wrap a layer of tinfoil on the outer surface to avoid light, mix 2.18g (10mmol) diphenyl sulfone and 4.29g (15mmol) 1,3-dibromo-5,5-dimethyl Add base hyne (DBH) into the three-necked flask, pump out three times quickly, slowly add 25mL of concentrated sulfuric acid dropwise under nitrogen atmosphere, stir at room temperature for 1 hour, slowly raise the temperature to 80°C, and continue the reaction for two hours before stopping. After cooling to room temperature, pour the reacted solution into 250 mL of ice water, filter the obtained white precipitate, wash with saturated brine and dry, and precipitate with dichloromethane / methanol to obtain a white solid 3BrDPS, white solid 3BrDPS3.59g , and the yield was 79.8%.
[0076] The common bromination method of aromatic compounds usually adopts Br 2 and N-bromos...
Embodiment 2
[0086] Synthesis of 4CzDPS
[0087]S30: Preparation of 1-((3,5-dibromophenylsulfone)-3,5-dibromobenzene (4BrDPS)
[0088] Take a 50mL three-neck flask, dry it thoroughly and wrap a layer of tinfoil on the outer surface to avoid light, mix 2.18g (10mmol) diphenyl sulfone and 5.72g (20mmol) 1,3-dibromo-5,5-dimethyl Genhyne was quickly added to the three-necked flask, and after pumping for three times, 30 mL of concentrated sulfuric acid was slowly added dropwise under nitrogen atmosphere. After stirring at room temperature for 1 hour, the temperature was slowly raised to 80°C, and the reaction was continued for 3 hours before stopping. After cooling to room temperature, the reacted solution was slowly poured into 300 mL of ice water to obtain a white precipitate, which was filtered out, washed with saturated brine, dried, and precipitated with dichloromethane / methanol to obtain 4.75 g of white solid 4BrDPS, which produced The rate is 89.6%;
[0089] The synthetic route is show...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com