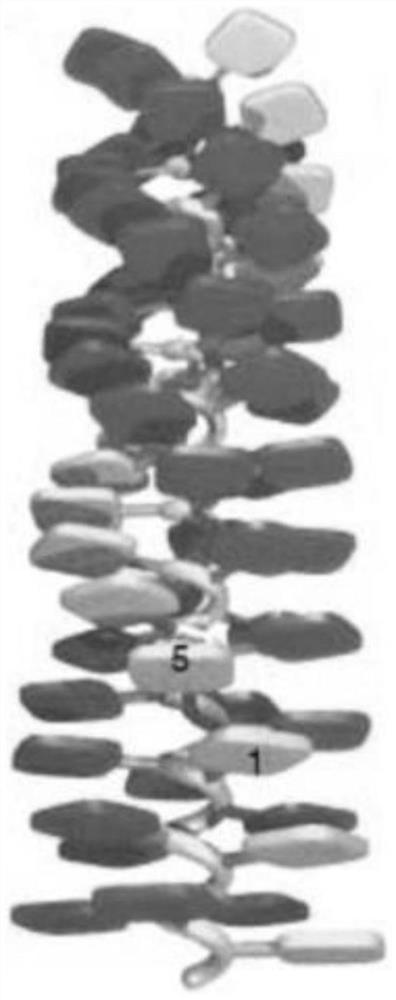
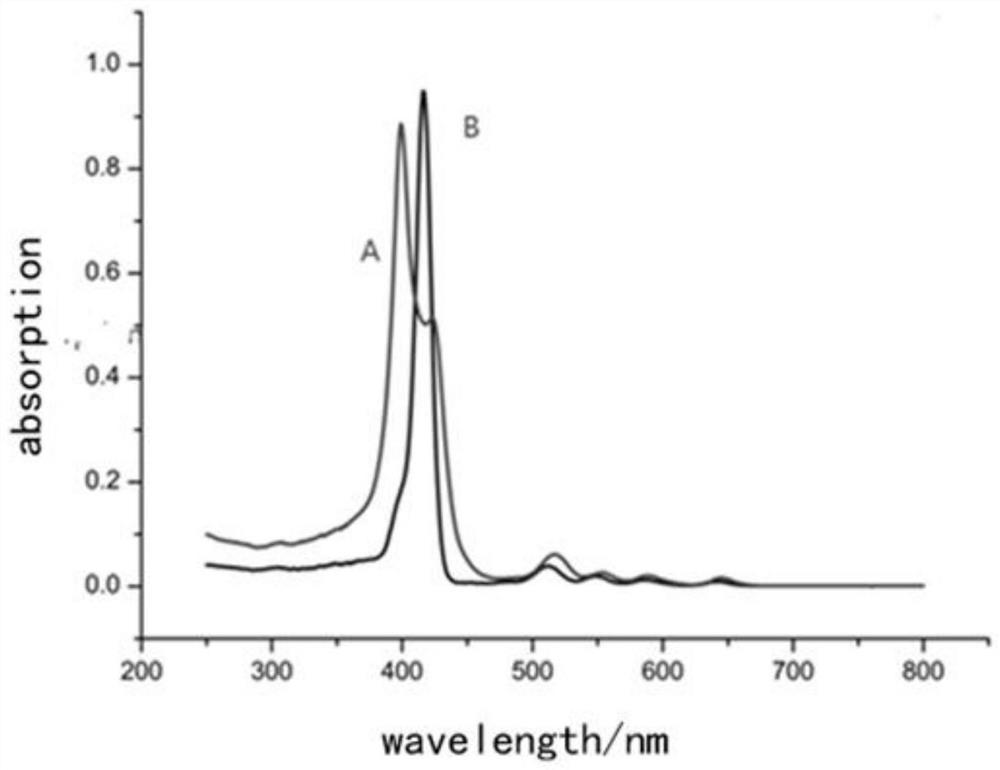
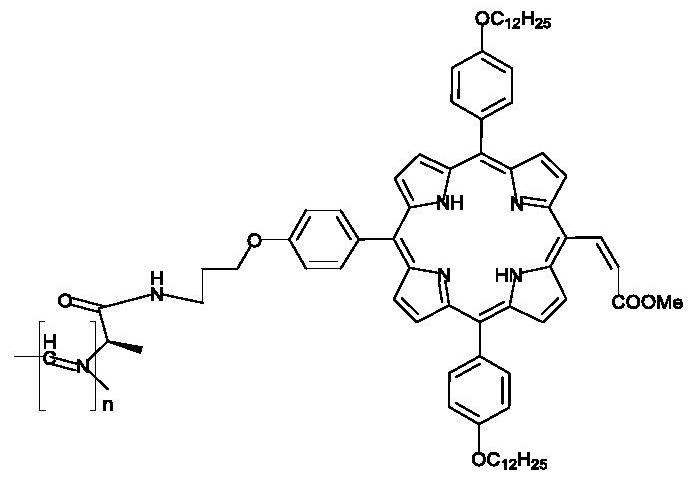
A kind of porphyrin class supramolecular helical polymer and its synthetic method
A synthesis method and supramolecular technology, which can be used in organic chemistry, photosensitive equipment, etc., can solve the problem of low light capture ability, and achieve the effect of high dye adsorption capacity, wide absorption spectrum, and wide application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1) Synthesis of Compound 3:
[0042] At room temperature, compound 1 (4.3784g, 20mmol), compound 2 (3.7842g, 20mmol), HOBt (2.9729g, 22mmol), DIPEA (3.48mL, 20mmol) and EDC.HCl (4.2174g, 22mmol) were added to the circle Bottom flask, then add 60ml of dichloromethane, react at 35°C for 6 hours, then extract 3 times with saturated aqueous citric acid solution, saturated aqueous sodium bicarbonate solution, and saturated aqueous sodium chloride solution, and collect organic phase, then dried with anhydrous sodium sulfate, filtered and evaporated, and the residue was purified by 300-400 mesh silica gel column chromatography (petroleum ether / ethyl acetate=1 / 1) to obtain 4.1g of compound 3. The yield was 49.1%. The resulting product is used 1 HNMR (CDCl 3 ,400MHz) for characterization, the following data were obtained: δ H 6.75(s,1H),5.22(s,1H),4.16(q,J=7.0Hz,1H),3.43(t,J=6.6Hz,2H),3.39(m,2H),2.08(q,J =6.5Hz, 2H), 1.45(s, 9H), 1.36(d, J=7.0Hz, 2H).
[0043] 2) Synthesi...
Embodiment 2
[0052] 1) Synthesis of compound 3:
[0053] At room temperature, compound 1 (5.4685g, 25mmol), compound 2 (4.2572g, 22.5mmol), HOBt (4.7566g, 32mmol), DIPEA (5.57mL, 32mmol) and EDC.HCl (6.1344g, 32mmol) were added to In the round-bottomed flask, then add 80ml of dichloromethane, react at 35°C for 7 hours, then extract 3 times with saturated aqueous citric acid solution, saturated aqueous sodium bicarbonate solution, and saturated aqueous sodium chloride solution, and collect The organic phase was then dried with anhydrous sodium sulfate, filtered and then rotary evaporated, and the residue was purified by 300-400 mesh silica gel column chromatography (petroleum ether / ethyl acetate=1 / 1) to obtain 5.9g of compound 3, the yield was 56.3%. The resulting product is compared and characterized by thin-layer chromatography with the above-mentioned Example 1.
[0054] 2) Synthesis of compound 4:
[0055] At room temperature, compound 3 (3g, 9.3mmol) was added to a round-bottomed fl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com