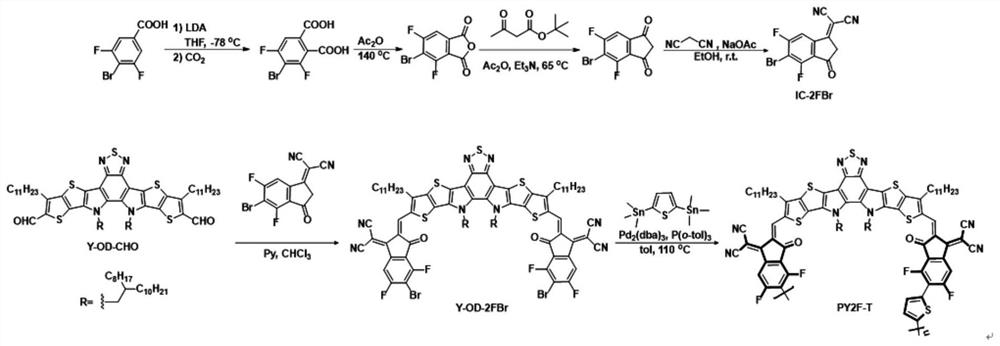
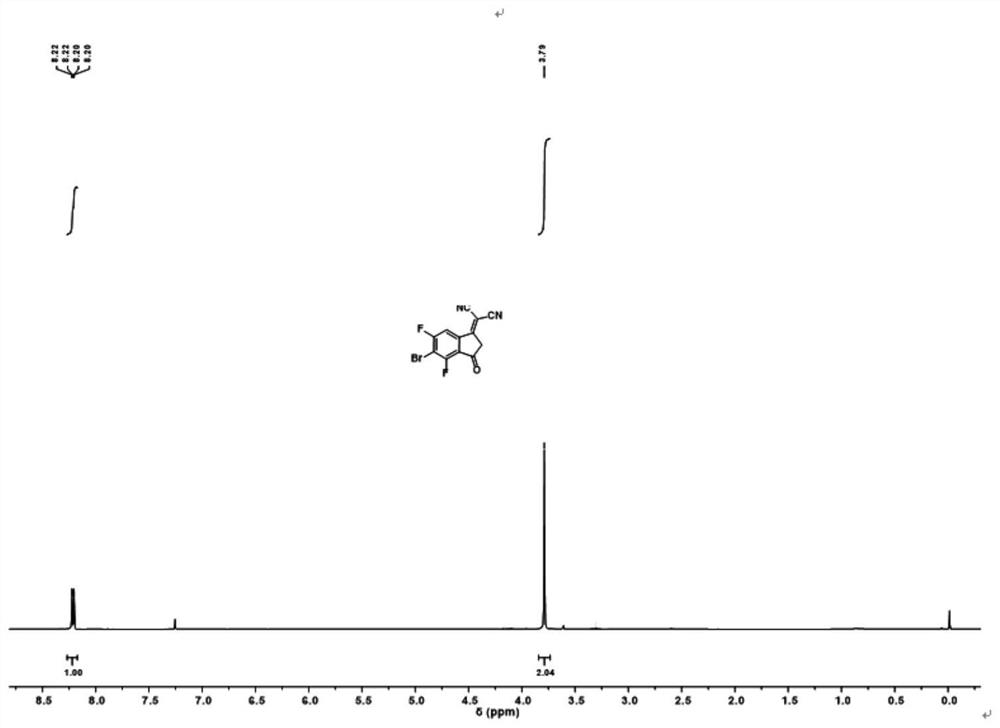
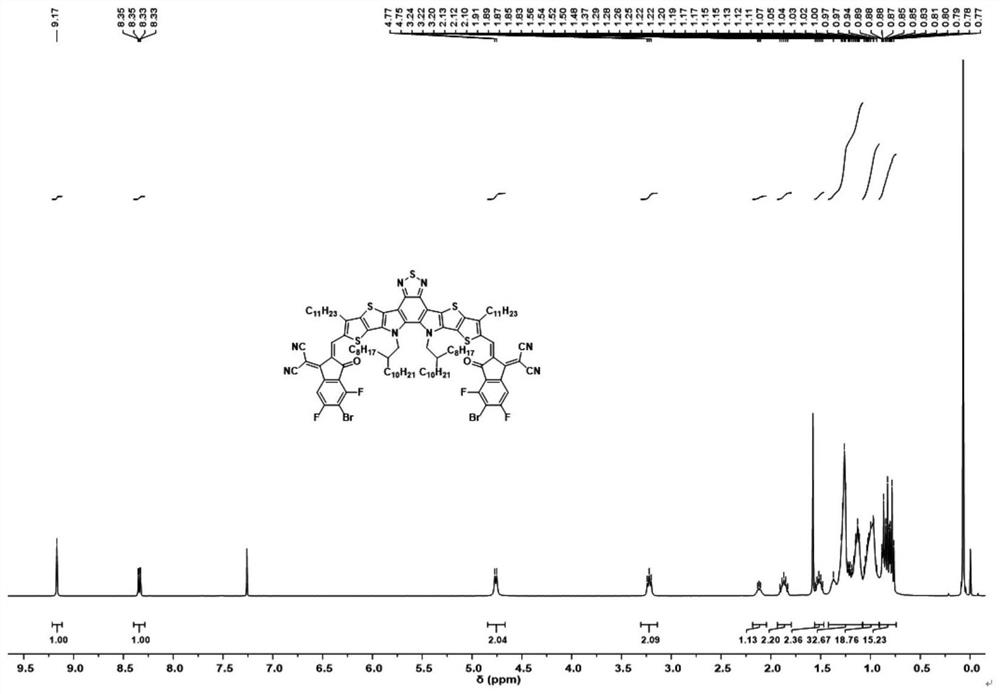
Fluorinated fused ring benzothiadiazole polymer acceptor material and preparation method thereof
A technology of benzothiadiazole and acceptor materials, which is applied in the field of organic solar cell material preparation, can solve the problems of low utilization rate of near-infrared photons and narrow absorption range, and achieve strong visible-near-infrared light absorption performance, high photoelectricity Conversion efficiency, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] R1 mentioned above is Ar is , R3 is EG is When , the preparation of the acceptor material is as follows:
[0096] (1) 4,7-dibromo-5,6-dinitrobenzothiadiazole and compound A are reacted by Stille coupling to obtain compound B:
[0097] Synthesis of Compound B: In a 250ml round bottom flask, weigh 4,7-dibromo-5,6-dinitrobenzothiadiazole (7.68g, 18mmol) and tributyl (6-undecylthiophene And[3,2-b]thiophen-2-yl)stannane (25.68g, 44mmol) was dissolved in 100ml of tetrahydrofuran, under the protection of argon, bistriphenylphosphine palladium dichloride (0.62g, 0.88mmol) was added in the system. The mixture was refluxed at 80°C for 20 hours. Cooled to room temperature, spin-dried THF, extracted with dichloromethane, spin-dried the solvent to obtain a crude product, separated and purified by silica gel column chromatography to obtain a rose-red solid (9.49 g), which is Compound B;
[0098] (2) Compound B, triphenylphosphine and o-dichlorobenzene undergo condensati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com