Methods and compositions for use of therapeutic t cells in combination with kinase inhibitors
A treatment method and inhibitor technology, applied in the field of preparing such engineered cells, can solve the problems of incompletely satisfactory methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0455] B. Cells and Cell Preparation for Genetic Engineering
[0456] Cells expressing receptors and administered by the provided methods include engineered cells. Genetic engineering generally involves introducing nucleic acids encoding recombinant or engineered components into cell-containing compositions, such as by retroviral transduction, transfection or transformation.
[0457] In some embodiments, the nucleic acid is heterologous, i.e., not normally present in the cell or a sample obtained from the cell, such as a nucleic acid obtained from another organism or cell, e.g., the nucleic acid is not normally present in the engineered cell and / or the organism from which such cells are derived. In some embodiments, the nucleic acid is not a naturally occurring nucleic acid such as one not found in nature, including nucleic acids comprising chimeric combinations of nucleic acids encoding various domains from multiple different cell types.
[0458] Cells are typically eukaryo...
Embodiment 1
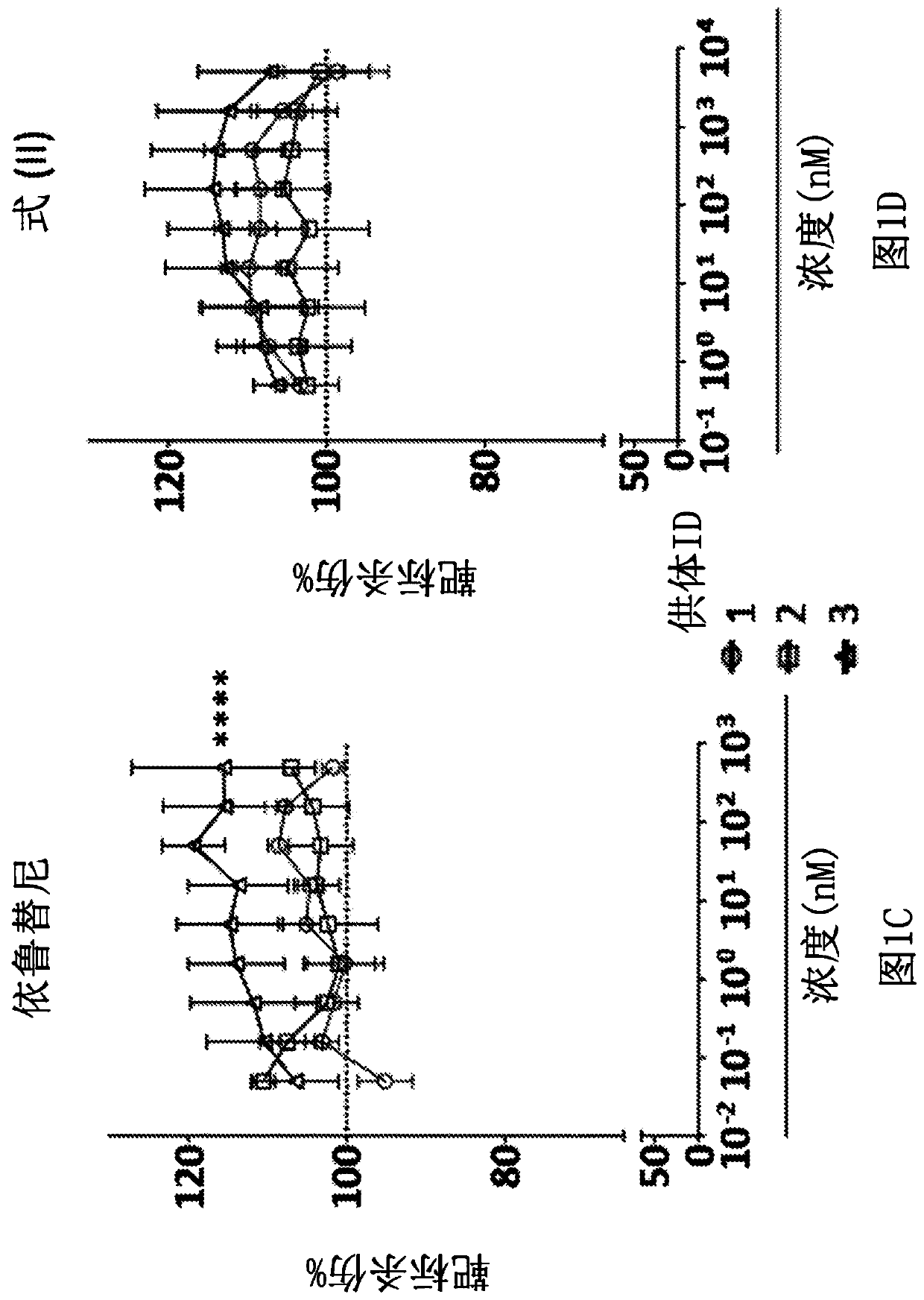
[0802] Example 1: Evaluation of the Phenotype and Function of CAR-Expressing T Cells in the Presence of Inhibitors of the TEC Family
[0803] The properties of CAR-expressing T cells in the presence of inhibitors of TEC family kinases, ibrutinib or a compound of formula (II) were evaluated in in vitro studies.
[0804]To generate CAR-expressing T cells, T cells were isolated from three healthy human donor subjects by immunoaffinity-based enrichment, and cells from each donor were activated and transduced with a viral vector encoding an anti-CD19 CAR. The CAR contains an anti-CD19 scFv, an Ig-derived spacer, a human CD28-derived transmembrane domain, a human 4-1BB-derived intracellular signaling domain, and a human CD3ζ-derived signaling domain. The CAR-encoding nucleic acid construct also includes a truncated EGFR (tEGFR) sequence used as a transduction marker, which is separated from the CAR sequence by a self-cleaving T2A sequence.
[0805] CAR-expressing CD4+ and CD8+ ce...
Embodiment 2
[0823] Example 2: Improving the anti-tumor activity of CAR-expressing T cells in the presence of inhibitors of TEC family kinases sex
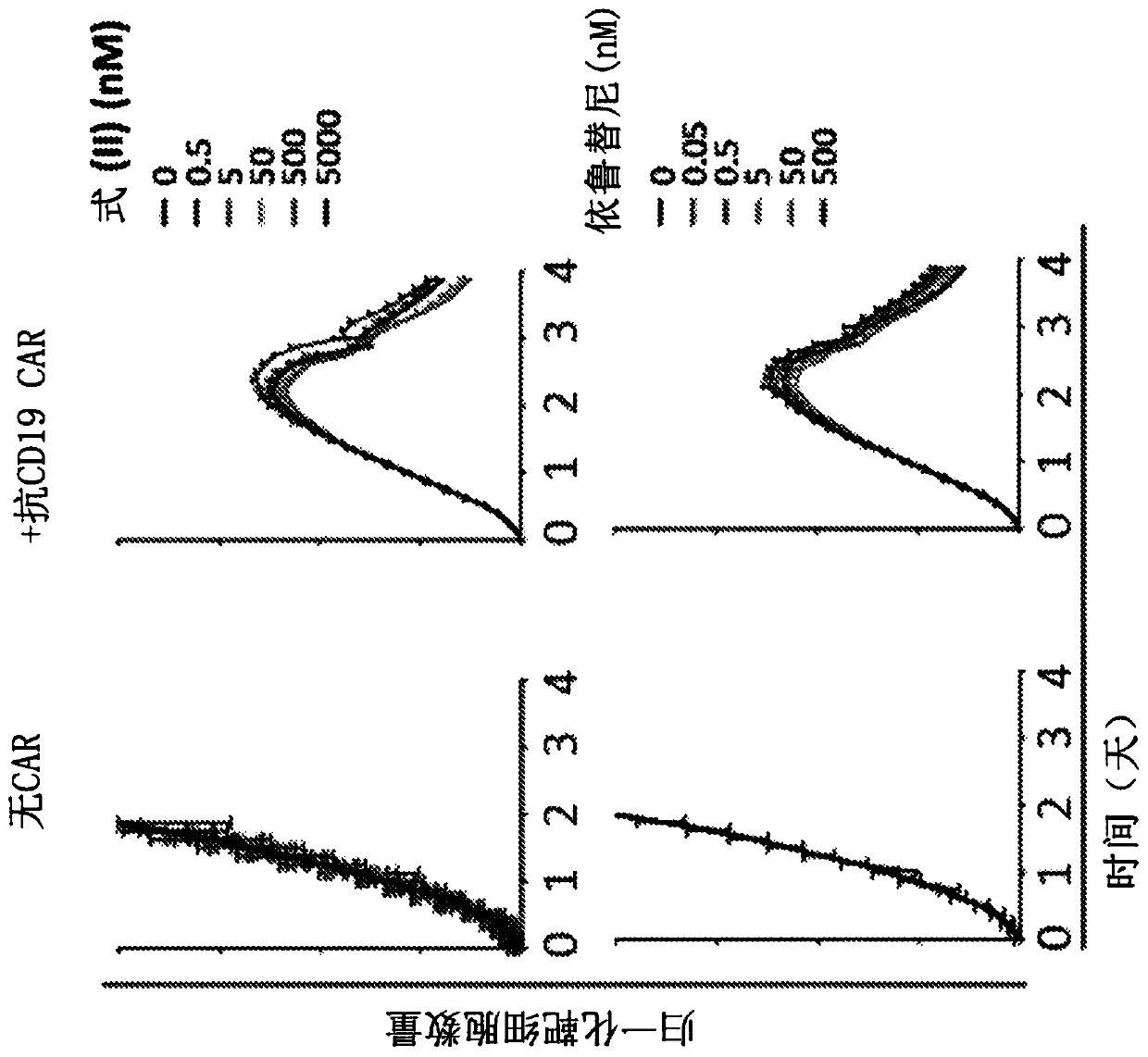
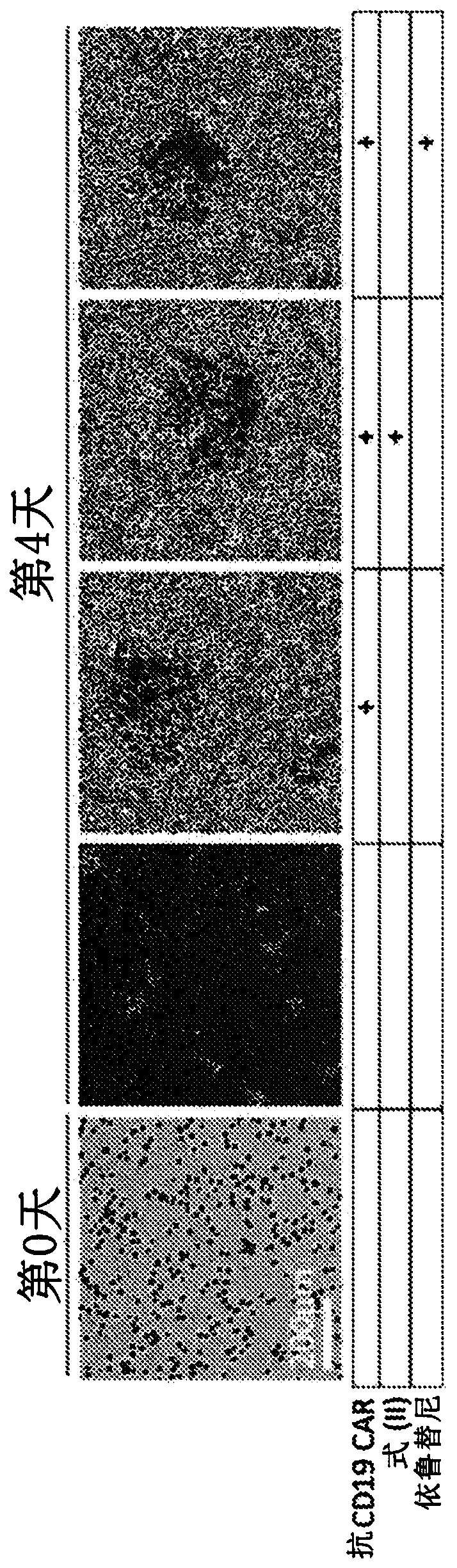
[0824] A disseminated tumor xenograft mouse model was generated by injecting NOD / Scid / gc- / - (NSG) mice with a CD19+Nalm-6 disseminated tumor cell line identified as resistant to BTK inhibition.
[0825] On day zero (0), NSG mice were injected intravenously with 5x 10 5 Nalm-6 cells expressing firefly luciferase. Beginning on day 4 and for the duration of the study mice were treated daily with vehicle control or ibrutinib, in each case by daily oral gavage (po) at 25 m / kg four times per day. To allow assessment of the effect of combination therapy with inhibitors, suboptimal doses of anti-CD19 CAR T cells from two different donors were administered at 5x10 on day 5. 5 / mouse Intravenous injection into mice. As a control, mice were given vehicle control or ibrutinib, but no CAR-T cells. Eight (N=8) mice were monitored per group.
[0826...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com