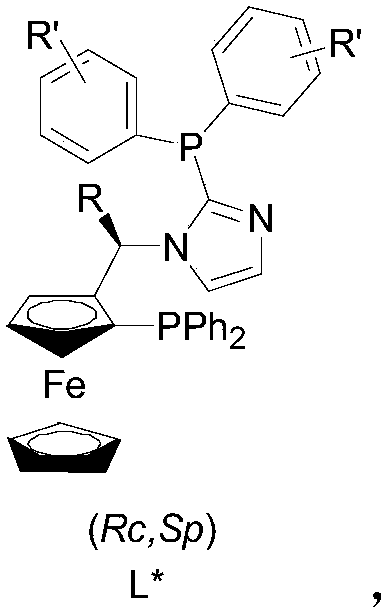
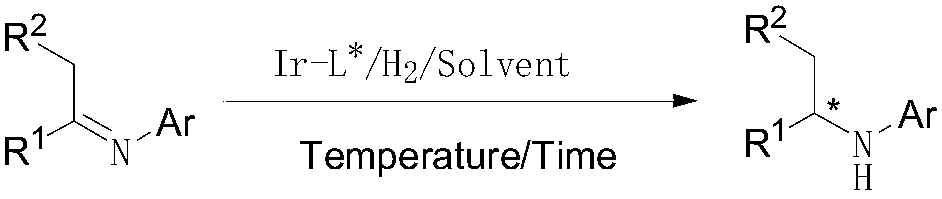Iridium/chiral diphosphine system catalyzed imine asymmetric hydrogenation method
A chiral bisphosphine ligand, a technology for catalyzing imines, applied in organic chemistry methods, chemical instruments and methods, catalysts for physical/chemical processes, etc., can solve problems such as high equipment requirements and difficulty in ligand synthesis, and achieve catalytic activity. High, Inexpensive, Mild Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The ligand synthesis method involved in the present invention is shown in the following reaction equation:
[0039]
[0040] Among them, (R c ,S p )-I a :R=Me, R'=H; (R c ,S p )-I b : R=Et, R'=H; (R c ,S p )-I c :R=Ph,R'=H etc
[0041] 1 Preparation of ligand (R c ,S p )-I a
[0042] Under the condition of argon, 1.32g (R c ,S p )-PPFA(I) and 1.63g of imidazole were dissolved in 15ml of dehydroacetic acid, heated to 80°C for 8 hours. After cooling, it was neutralized with excess saturated sodium bicarbonate solution, then extracted with dichloromethane (3×50ml), the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and desolvated. The crude product was analyzed by column chromatography (n-hexane Alkane / ethyl acetate / triethylamine=10 / 10 / 1) to obtain 1.24 g of brown crystal II, yield 89%.
[0043] Under the condition of argon, 0.23g II was dissolved in 15ml dry ether, and 0.47ml n-BuLi was slowly added dropw...
Embodiment 2
[0063] The hydrogenation reaction was carried out in a 200ml autoclave. First replace the reactor with nitrogen three times, then inject 52g of imine (generated by 2-methyl-6-ethylaniline and methoxyacetone) into the reactor, and then inject 0.5ml of in-situ coordinated catalyst Ir -I a (S / C=5×10 5 ). Then replace with hydrogen three times, pressurize to 50bar with hydrogen, raise the temperature to 80°C for 3h, cool down, release the pressure, open the kettle, and the reaction conversion rate is greater than 99% according to GC analysis, and 50g (S)-NAA is obtained by distillation under reduced pressure. 95%, HPLC analysis ee value is 91%.
[0064] The liquid phase and NMR spectrum data are as follows:
[0065] HPLC (OJ-H, n-hexane / i-PrOH=98 / 2, 1.0ml / min, 254nm, 40°C):t R (minor)=3.9min,t R (major) = 4.3min. 1 H NMR (400MHz, CDCl 3 ):7.02(dd, J=7.6,15.2Hz,2H),6.89(t,J=7.6Hz,1H),3.36-3.40(m,6H),2.67(q,J=7.6Hz,2H),2.31 (s, 3H), 1.25 (t, J = 7.6Hz, 3H), δ = 1.20 (d, J =...
Embodiment 3
[0069] Other conditions are identical with example 2, catalyst Ir-I a Change to Ir-I b , GC analysis reaction conversion rate is greater than 99%, HPLC analysis ee value is 85%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap