Preparation method and application of diosgenin derivatives containing 1,3,4 oxadiazole or 1,3,4 thiadiazole fragments
A technology of diosgenin and derivatives, which is applied in the field of natural medicine and medicinal chemistry, can solve the problems of large side effects and limited use, and achieve significant anti-lung cancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
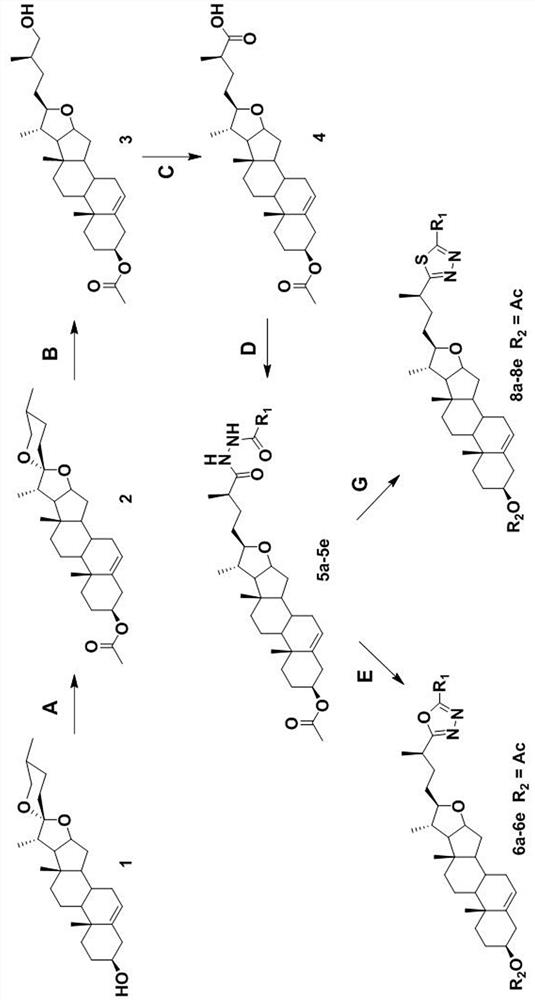
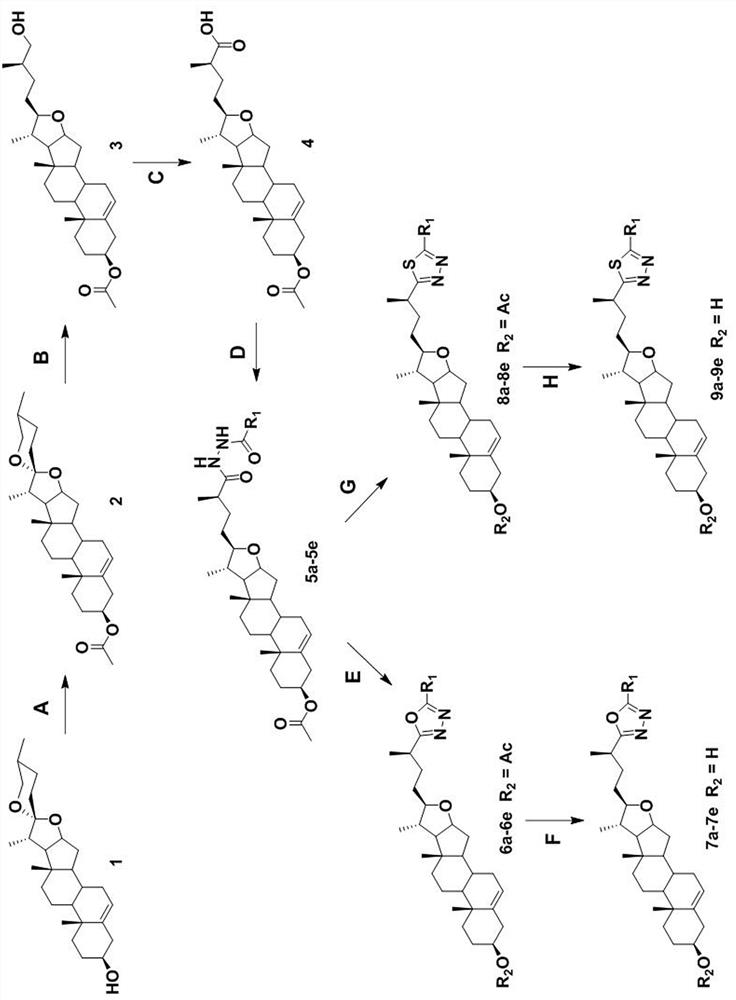
[0025] Specific Embodiment 1: In this embodiment, diosgenin derivatives containing 1,3,4 oxadiazole or 1,3,4 thiadiazole fragments have a structural formula of R 1 It is an alkyl group containing 1 to 8 carbon atoms, an aromatic ring containing 5 to 12 carbon atoms, an alkoxy-substituted aromatic ring or an aromatic heterocyclic ring containing 5 to 12 carbon atoms, and the aromatic heterocyclic ring includes 1 to 3 heteroatoms of N, O or S, R 2 For H- or acetyl.
specific Embodiment approach 2
[0026] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the R 1 is methyl, phenyl, p-methoxyphenyl, 1-naphthyl or 3-pyridyl.
[0027] Other steps are the same as in the first embodiment.
specific Embodiment approach 3
[0028] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is: the preparation method of diosgenin derivatives containing 1,3,4 oxadiazole fragments is completed according to the following steps:
[0029] said Middle R 2 When it is an acetyl group, it is prepared according to the following steps: Diosgenin is in CH 2 Cl 2And react with acetic anhydride in pyridine solvent, obtain intermediate I, intermediate I is in CH 2 Cl 2 and glacial acetic acid by NaBH 3 CN was reduced to intermediate II, which was then oxidized by Jones reagent, tetrahydrofuran and acetone to intermediate III, followed by O-benzotriazole-N,N,N′,N′-tetramethylurea tetrafluoroborate As a coupling catalyst, N,N-diisopropylethylamine and dichloromethane, with acetylhydrazide, benzohydrazide, p-methoxybenzohydrazide, 1-naphthohydrazide or 3-pyridineformyl Hydrazine condensation to obtain intermediate IV; and then through POCl 3 Dehydration and cyclizati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com