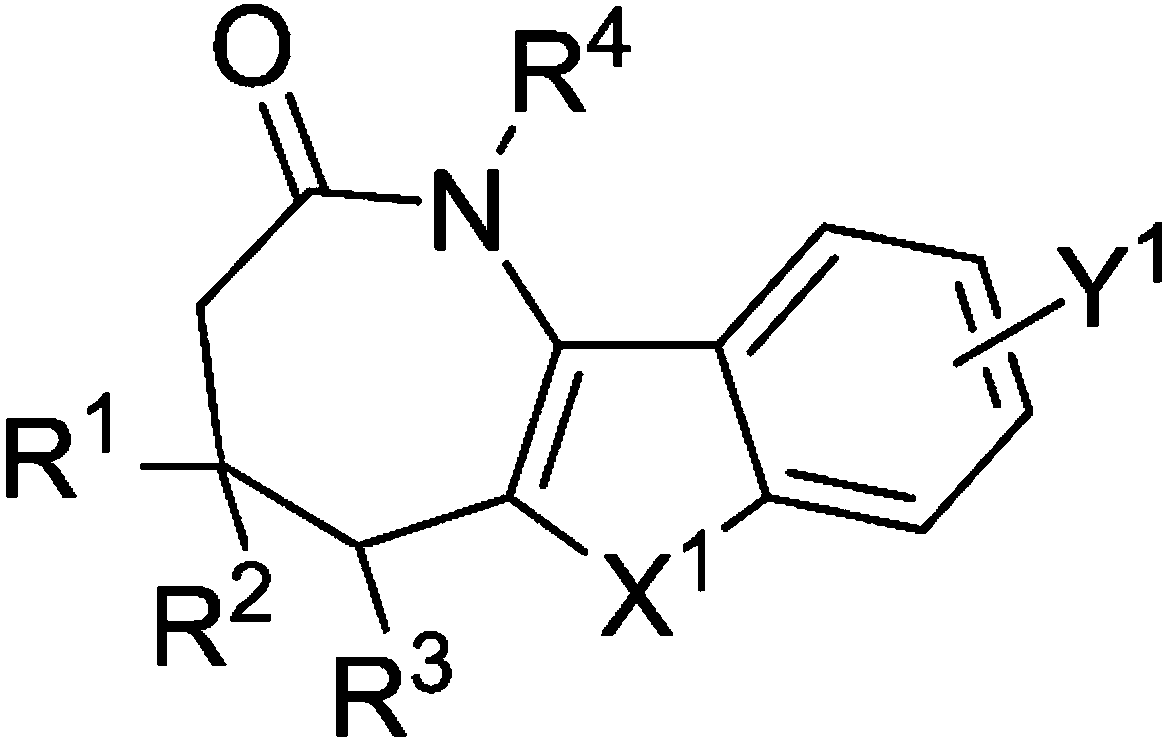
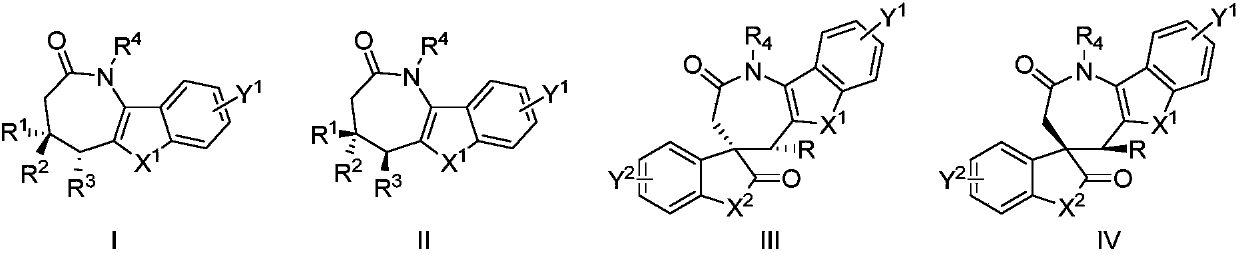
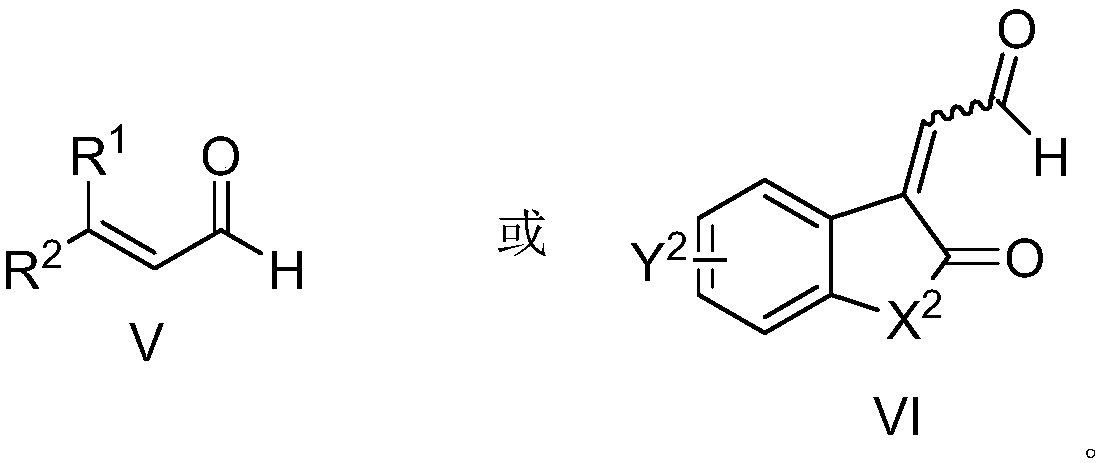
Aza*one compound and preparation method thereof
A compound, azaketone technology, applied in the field of tumor treatment drugs, can solve problems such as slow development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] The pre-dried reaction tube was lowered to room temperature under vacuum, and nitrogen heterocyclic carbene VIII-1 (Ar 1 =3,5-(CF 3 ) 2 C 6 h 3 ,R=H,Ar 2 =Ph) (0.01mmol, 10mol%), potassium acetate (0.02mmol, 20mol%), unsaturated imine VII-1 (0.1mmol, 1.0equiv), inject 2-hexenal V-1 (0.2mmol, 2.0 equiv), add 2.0 milliliters of anhydrous 1,4-dioxane under nitrogen protection, move to 40 ° C oil bath and stir, until the thin layer chromatography control shows that the imine disappears (generally 12 hours), the reaction solution is concentrated, and the column After chromatographic separation and purification, the compound shown in I-1 was obtained.
[0088] Its structural formula is:
[0089]
[0090] The experimental data of I-1 are as follows:
[0091] 44mg,>20:1d.r.,94%yield.Yellow oil.R f =0.2(petroleum ether / ethylacetate 10:1) HPLC analysis: 99%ee[Daicel CHIRALPAKIA column, 20°C, 254nm, hexane / i-PrOH=90:10, 1.0mL / min, 13.4min(major), 19.7min(minor)]; 1 H...
Embodiment 2
[0093] The pre-dried reaction tube was lowered to room temperature under vacuum, and nitrogen heterocyclic carbene VIII-2 (Ar 1 =3,5-(CF 3 ) 2 C 6 h 3 ,R=H,Ar 2 =2-iPrC 6 h 4 ) (0.01mmol, 10mol%), potassium acetate (0.02mmol, 20mol%), unsaturated imine VII-1 (0.1mmol, 1.0equiv), injected enaldehyde V-2 (0.2mmol, 2.0equiv), nitrogen protection Add 2.0 ml of anhydrous 1,4-dioxane, move to 40°C oil bath and stir until the thin layer chromatography shows that the imine disappears (generally 12 hours), the reaction solution is concentrated, and after separation and purification by column chromatography The compound shown in I-2 was obtained.
[0094] Its structural formula is:
[0095]
[0096] The experimental data of I-2 are as follows:
[0097] 50.4mg, 9:1d.r., 93% yield. Yellow oil; HPLC analysis: 99%ee[Daicel CHIRALPAK IA column, 20°C, 254nm, hexane / i-PrOH=90:10, 1.0mL / min, 12.3min(major), 16.3min(minor)]; 1 H NMR (400MHz, CDCl 3)δ7.96(d, J=8.4Hz, 3H), 7.56-7.5...
Embodiment 3
[0099] The pre-dried reaction tube was lowered to room temperature under vacuum, and nitrogen heterocyclic carbene VIII-2 (Ar 1 =3,5-(CF 3 ) 2 C 6 h 3 ,R=H,Ar 2 =2-iPrC 6 h 4 ) (0.01mmol, 10mol%), potassium acetate (0.02mmol, 20mol%), unsaturated imine VII-1 (0.1mmol, 1.0equiv), injected enaldehyde V-3 (0.2mmol, 2.0equiv), nitrogen protection Add 2.0 ml of anhydrous 1,4-dioxane, move to 40°C oil bath and stir until the thin layer chromatography shows that the imine disappears (generally 12 hours), the reaction solution is concentrated, and after separation and purification by column chromatography The compound shown in I-3 was obtained.
[0100] Its structural formula is:
[0101]
[0102] The experimental data of I-3 are as follows:
[0103] 40mg, 9:1d.r., 79% yield.Yellow solid, m.p.185-187℃.R f =0.2(petroleumether / ethyl acetate 10:1); HPLC analysis: 98%ee[Daicel CHIRALPAK IA column, 20°C, 254nm, hexane / i-PrOH=90:10, 1.0mL / min, 10.3min(major), 13.9min(minor)];...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com