Hybrid pseudo-estrogen and anti-estrogen disruptor recognition method based on enhanced sampling molecular dynamics simulation
A molecular dynamics, anti-estrogen technology, applied in informatics, computational theoretical chemistry, instrumentation, etc., can solve problems such as limitations, and achieve the effect of low cost and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Molecular dynamics simulation of ERα
[0067] The human ERα structure was obtained from the ERα protein structure with PDB code 3erd obtained from the Protein Data Bank, and was checked for structural integrity and repaired by default residues under the Swiss-PdbViewer software. Ligand small molecules, including standard substances and endocrine disruptors bisphenols commonly found in the environment, are structurally optimized and docked with the receptor using the Surflex-Dock module in SYBYL 7.3 to form a ligand-receptor complex. The complex was simulated using the well-recognized enhanced sampling molecular dynamics simulation method—metadynamics simulation method—for enhanced sampling molecular dynamics simulation. The molecular simulation software used were gromacs and plumed software packages. Metadynamics simulations were carried out with the distances between L544 and E380 and between L544 and M522 in ERα as ensemble variables. Each metadynamics simu...
Embodiment 2
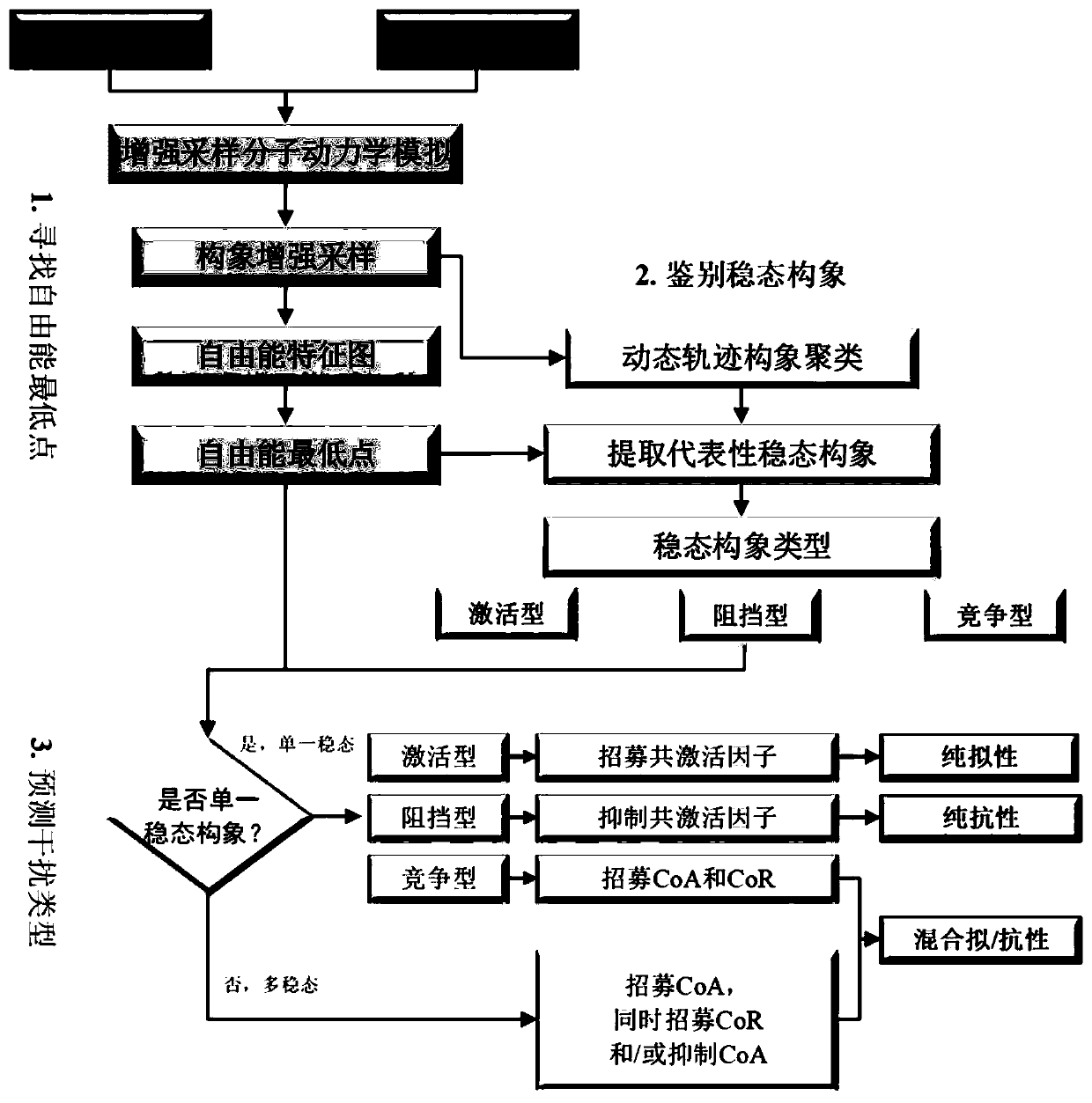
[0068] Example 2: Analysis of Molecular Dynamics Simulation Results
[0069]The molecular simulation trajectories obtained by the metadynamics simulation carried out in the present invention are used for further analysis. The dihedral angle formed by the α carbon atoms on the four amino acids M543, L539, A350 and L354 of ERα is used as set variable 1 (CV1, in radians), and the angle formed by the α carbon atoms on the three amino acids L539, M534 and M522 is CV2 (unit is radian), draw the characteristic map of free energy (Free Energy, unit is kJ / mol), describe the position of H12, obtain the lowest point of global and local free energy, and obtain the lowest point of free energy through conformational clustering. The representative conformation is the representative steady state conformation. According to the stable position of H12 in the representative steady-state conformation, the type of each steady-state conformation was judged. Finally, according to the type and quant...
Embodiment 3
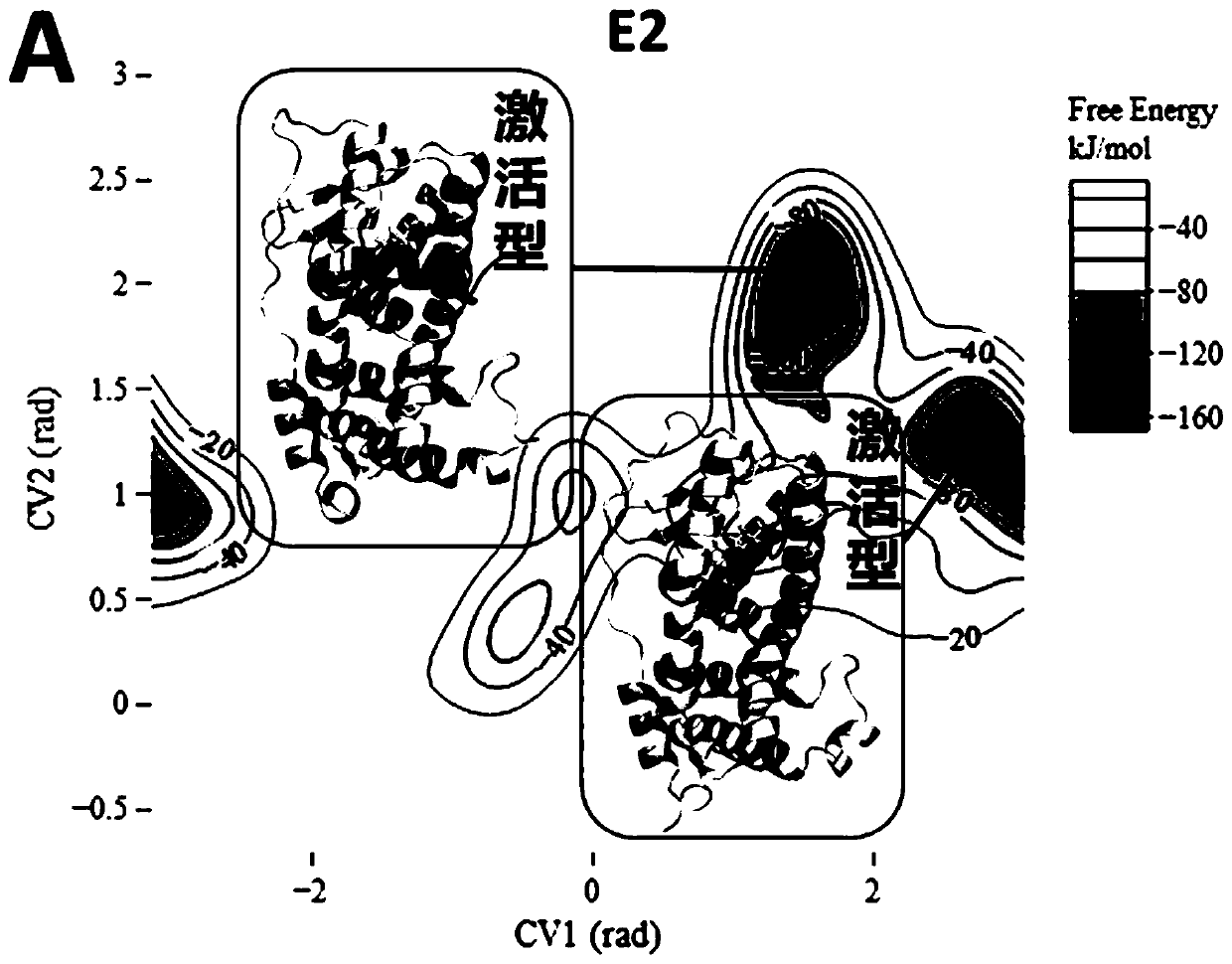
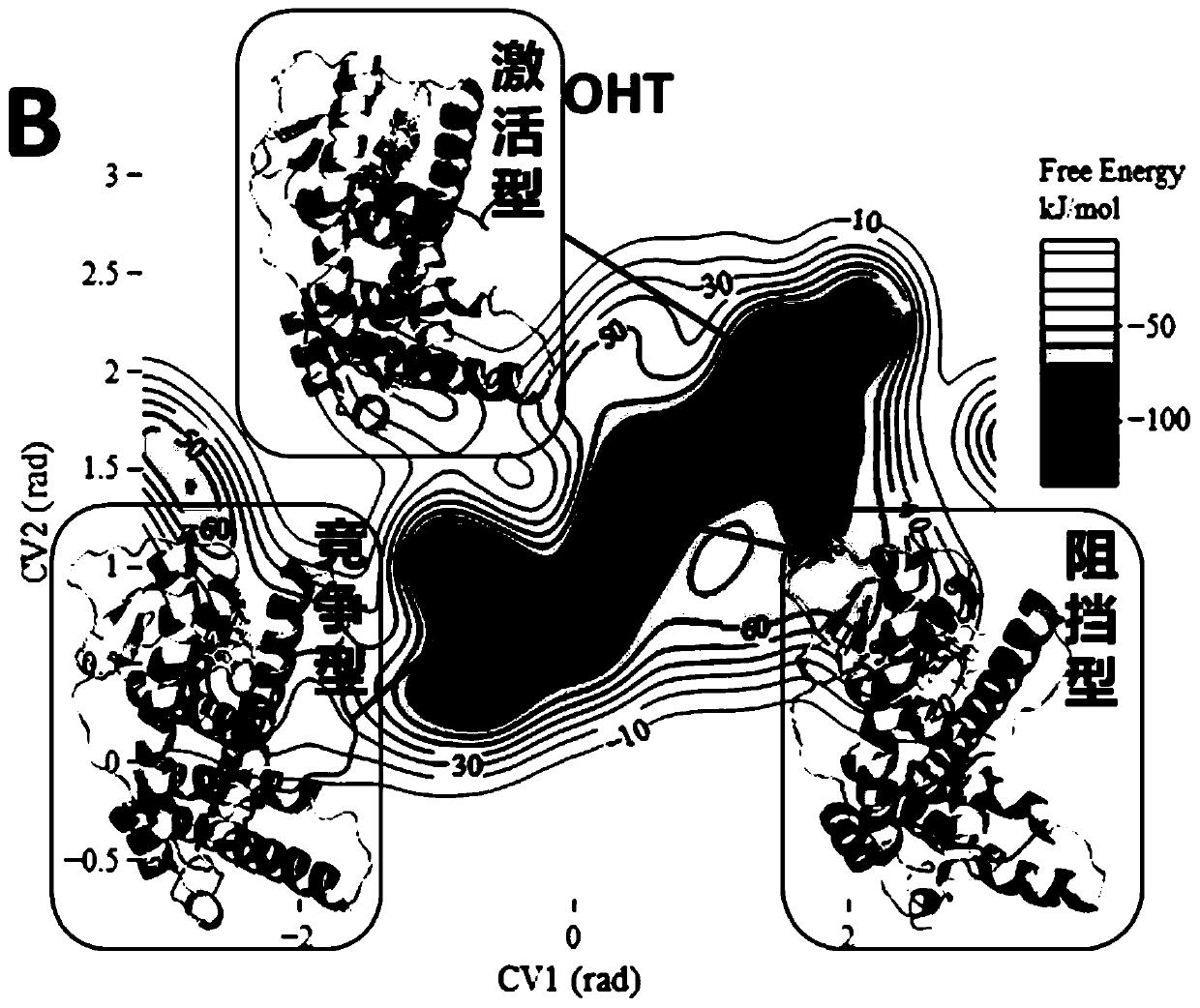
[0070] Example 3: Analysis of the results of standard substances E2 and OHT
[0071] For the pure pseudo-standard material E2, the metadynamics simulation results show that the steady-state conformations of E2-ERα are all activated conformations ( Figure 2A ), which is consistent with the reported crystal structure of E2-ERα. The activated conformation of H12 forms a "steric hindrance" effect to selectively recruit CoA. Therefore, under the action of the pure mimetic compound E2, ERα forms an activated conformation, which leads to the selective recruitment of CoA by the receptor, which in turn leads to transcriptional activation and the generation of pure mimetic effects. For OHT of mixed pseudo- and resistant compounds, metadynamics simulation results show that OHT-ERα has multiple types of steady-state conformations ( Figure 2B ). OHT-ERα has three types of steady-state conformations: activation, blocking and competition, and the blocking and competition conformations a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com