Method for determining pharmacokinetics of axitinib and method for predicting therapeutic effect of axitinib based on pharmacokinetics of axitinib
A technology of pharmacokinetics and determination methods, applied in chemical property prediction, biochemical equipment and methods, microbial measurement/testing, etc., can solve problems such as inapplicability of axitinib
Pending Publication Date: 2019-12-31
YAMAGUCHI UNIV +1
View PDF6 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0008] However, the technology disclosed in Patent Document 2 is related to the epidermal growth factor receptor tyrosine kinase inhibitor drug erlotinib, and this technology is not a model for directly predicting the therapeutic effect of erlotinib, but only uses ABCB1 complex pharmacokinetic prediction, etc., so this technology cannot be applied to axitinib
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
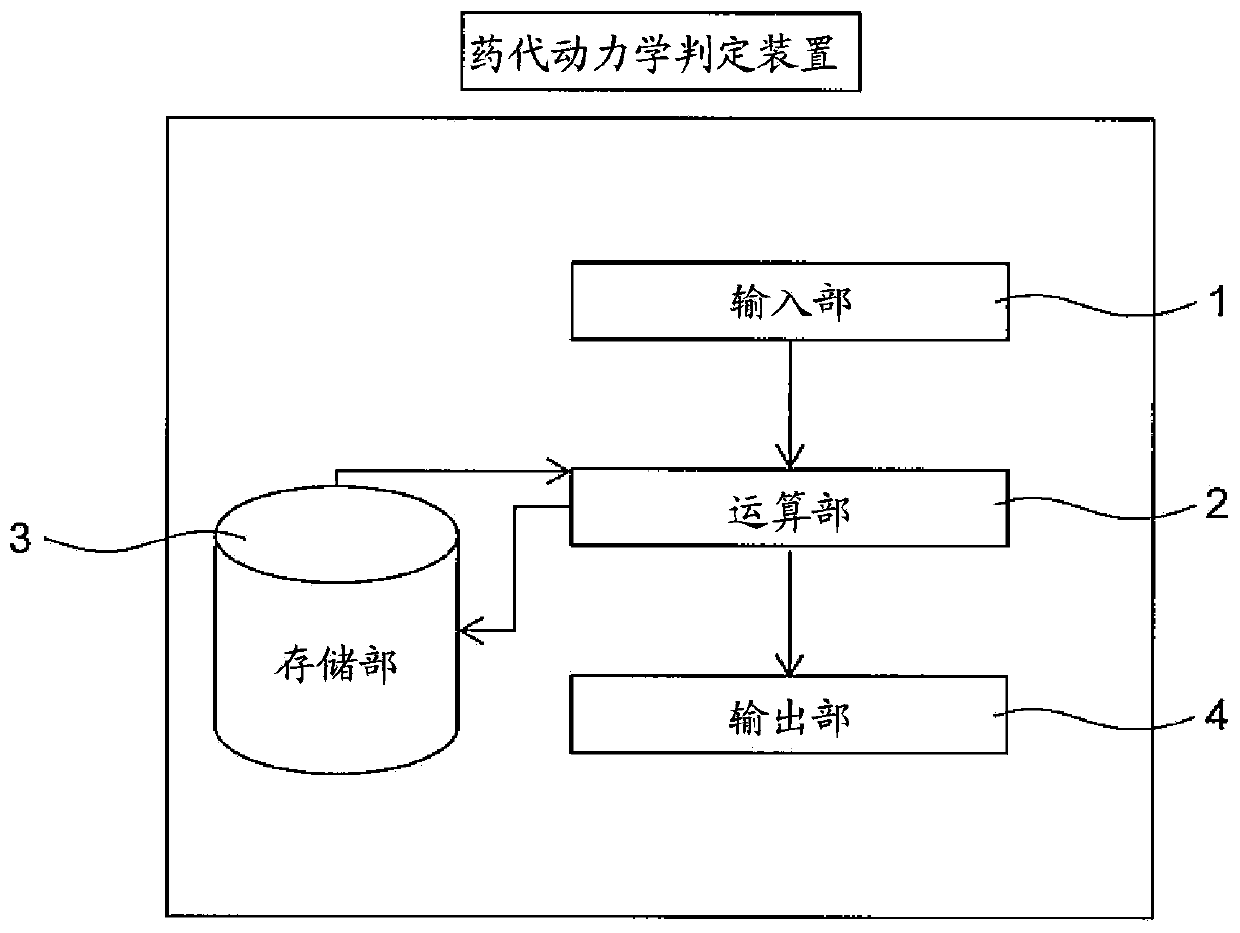
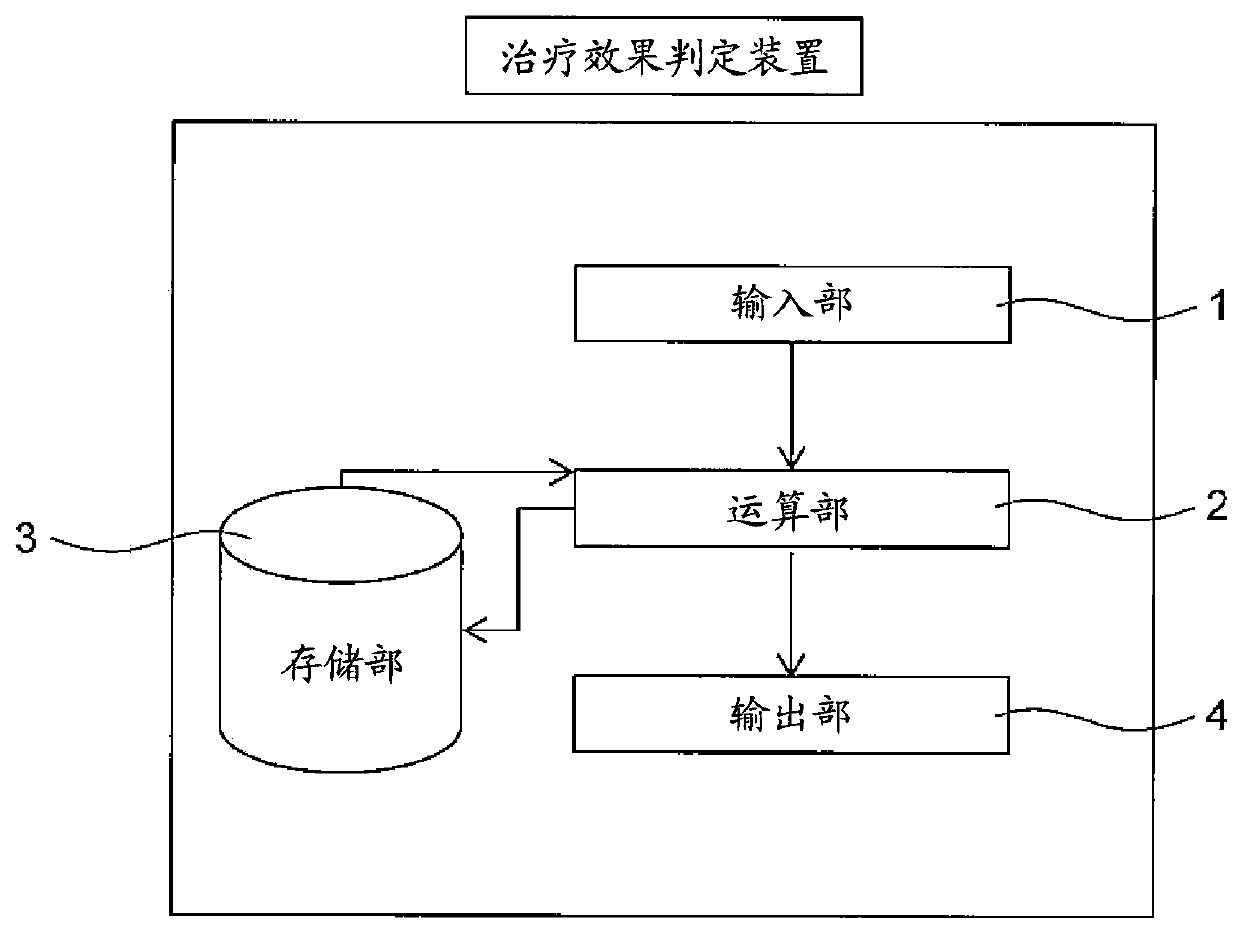
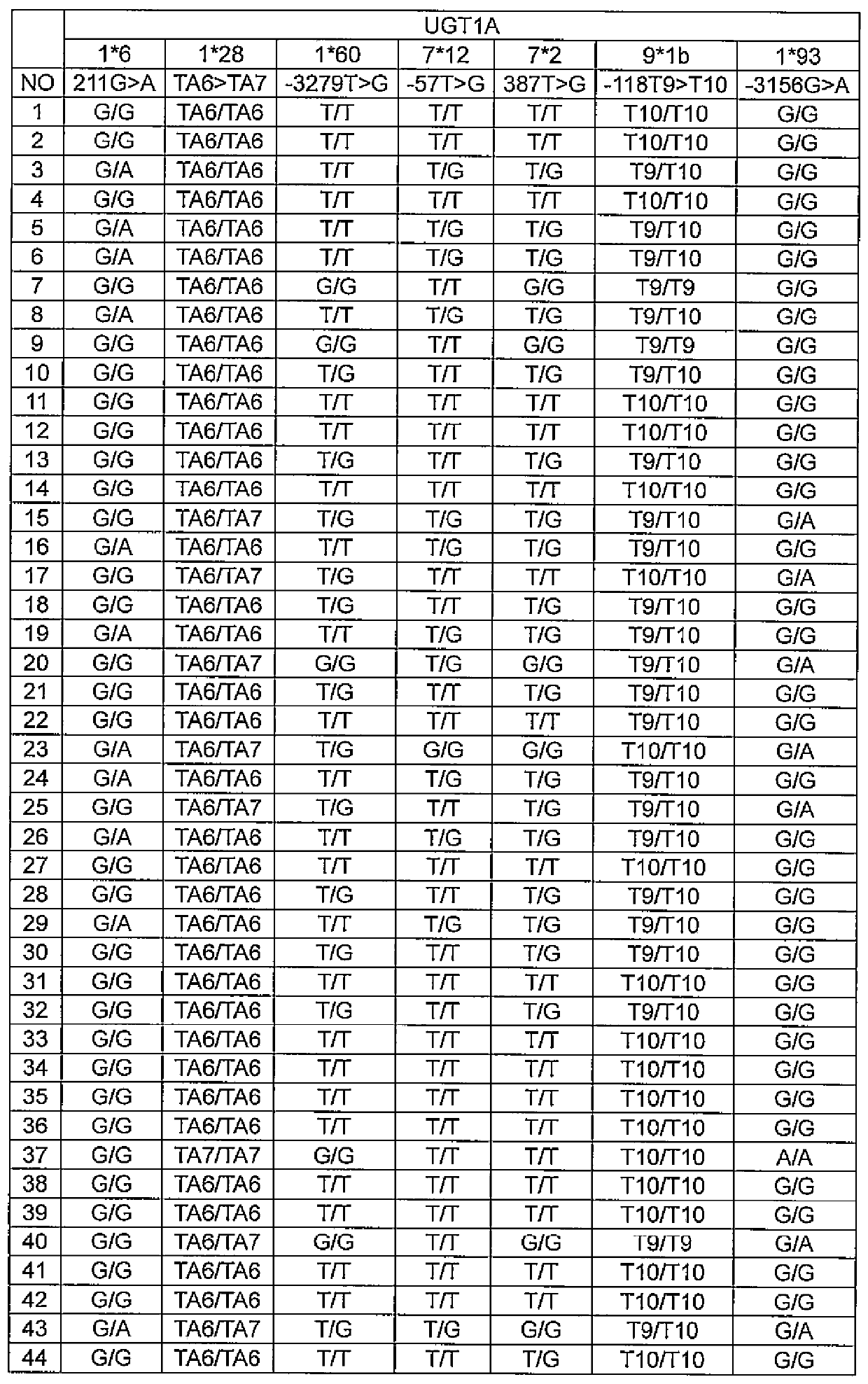
[0156] In this example, using a regression model with relevant gene polymorphism information and patient background factors as variables, a blood concentration-time curve for predicting the therapeutic effect and side effects of axitinib was established The model of the area under (AUC, one of the pharmacokinetic analysis parameters).
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
In the present invention, the pharmacokinetics of axitinib are easily determined and the therapeutic effect of axitinib are predicted. A method for determining the pharmacokinetics of axitinib that includes a step for calculating predictive pharmacokinetic parameters of axitinib using specific genetic polymorphisms and background factors relating to a subject.
Description
technical field [0001] The present invention relates to a method for judging the pharmacokinetics of axitinib that can be applied to unresectable or metastatic renal cell carcinoma, and a method for predicting the therapeutic effect of axitinib based on the pharmacokinetics of axitinib. Background technique [0002] Axitinib is an oral tyrosine kinase inhibitor that selectively inhibits vascular endothelial growth factor receptor (VEGFR)-1, vascular endothelial growth factor receptor-2, and vascular endothelial growth factor receptor-3 . Incidentally, VEGFR-1, VEGFR-2, and VEGFR-3 are considered to be involved in tumor growth, cancer invasion (tumor spread), and metastasis. It is also believed that axitinib can selectively inhibit VEGFR-1, VEGFR-2, and VEGFR-3, thereby inhibiting angiogenesis and lymphangiogenesis, and inhibiting tumor proliferation and metastasis, showing anti-tumor activity. [0003] Currently, axitinib is used as a molecularly targeted drug for unresect...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C12Q1/6886C12M1/34G01N33/53
CPCC12M1/34G01N33/53C12Q1/6886C12Q2600/106C12Q2600/156G16B20/20G16C20/30
Inventor 松山豪泰浜本義彦藤田悠介山本義明恒富亮一大场光芳山野博文石川有希华
Owner YAMAGUCHI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com